|
Antisolvent
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/L solution) or molal (mol/kg solvent) and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes. Quantifying ionic strength The molar ionic strength, ''I'', of a solution is a function of the concentration of ''all'' ions present in that solution. :I = \begin\frac\end\sum_^ c_i z_i^ where one half is because we are including both cations and anions, ''c''i is the molar concentration of ion i (M, mol/L), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Interactions
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
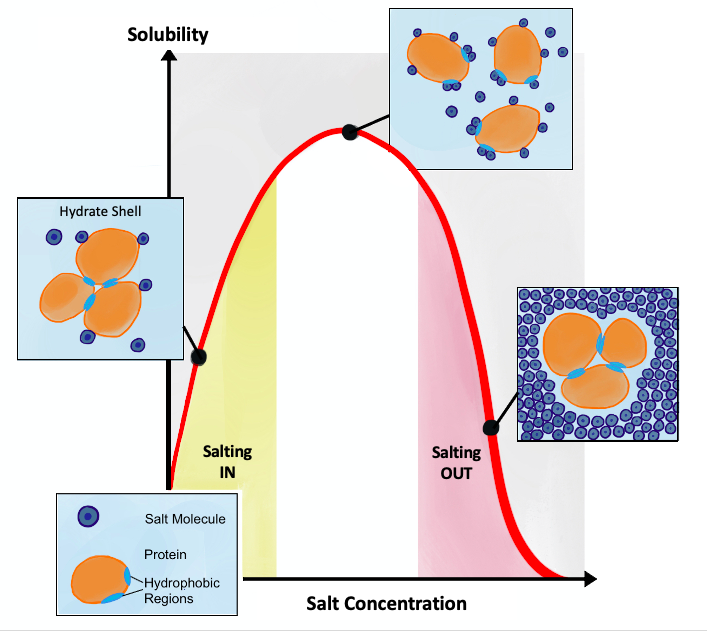
Hofmeister Series
The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins. Hofmeister discovered a series of salts that have consistent effects on the solubility of proteins and (it was discovered later) on the stability of their secondary and tertiary structure. Anions appear to have a larger effect than cations, and are usually ordered : \mathrm (This is a partial listing; many more salts have been studied.) The order of cations is usually given as : \mathrm The mechanism of the Hofmeister series is not entirely clear, but does not seem to result from changes in general water structure, instead more specific interactions between ions and proteins and ions and the water molecules directly contacting the proteins may be more important. Re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Sulfate Precipitation
Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentration. Properties Ammonium sulfate is an inorganic salt with a high solubility that disassociates into ammonium () and sulfate () in aqueous solutions. Ammonium sulfate is especially useful as a precipitant because it is highly soluble, stabilizes protein structure, has a relatively low density, is readily available, and is relatively inexpensive. Mechanism Ammonium sulfate, as well as other neutral salts, will stabilize proteins by preferential solvation. Proteins are usually stored in ammonium sulfate because it inhibits bacterial growth. With the addition of ammonium sulfate, proteins unfolded by denaturants can be pushed into their native conformations. This can be seen with the folding of recombinant proteins. The solubility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salting In
Salting in refers to the effect where increasing the ionic strength of a solution increases the solubility of a solute, such as a protein. This effect tends to be observed at lower ionic strengths. Protein solubility is a complex function of physicochemical nature of the protein, pH, temperature, and the concentration of the salt used. It also depends on whether the salt is kosmotropic, whereby the salt will stabilize water. The solubility of proteins usually increases slightly in the presence of salt, referred to as "salting in". However, at high concentrations of salt, the solubility of the proteins drop sharply and proteins can precipitate out, referred to as "salting out". Anionic interactions Initial salting in at low concentrations is explained by the Debye–Huckel theory. Proteins are surrounded by the salt counterions (ions of opposite net charge) and this screening results in decreasing electrostatic free energy of the protein and increasing activity of the solvent, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Precipitation
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent. General principles The solubility of proteins in aqueous buffers depends on the distribution of hydrophilic and hydrophobic amino acid residues on the protein's surface. Hydrophobic residues predominantly occur in the globular protein core, but some exist in patches on the surface. Proteins that have high hydrophobic amino acid content on the surface have low solubility in an aqueous solvent. Charged and polar surface residues interact with ionic groups in the solvent and increase the solubility of a protein. Knowledge o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/L solution) or molal (mol/kg solvent) and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes. Quantifying ionic strength The molar ionic strength, ''I'', of a solution is a function of the concentration of ''all'' ions present in that solution. :I = \begin\frac\end\sum_^ c_i z_i^ where one half is because we are including both cations and anions, ''c''i is the molar concentration of ion i (M, mol/L), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Centrifugation
Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate away from the axis of the centrifuge, while the less dense components of the mixture migrate towards the axis. Chemists and biologists may increase the effective gravitational force of the test tube so that the precipitate (pellet) will travel quickly and fully to the bottom of the tube. The remaining liquid that lies above the precipitate is called a supernatant or supernate. There is a correlation between the size and density of a particle and the rate that the particle separates from a heterogeneous mixture, when the only force applied is that of gravity. The larger the size and the larger the density of the particles, the faster they separate from the mixture. By applying a larger effective gravitational force to the mixture, like a ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as ''oversize'' and the fluid that passes through is called the ''filtrate''. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as ''blinding''. The size of the largest particles that can successfully pass through a filter is called the effective ''pore size'' of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles (depending on the pore size, filter thickness and biological activity). Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms. Filtration is als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantities in seawater. The open ocean has about of solids per liter of sea water, a salinity of 3.5%. Salt is essential for life in general, and saltiness is one of the basic human tastes. Salt is one of the oldest and most ubiquitous food seasonings, and is known to uniformly improve the taste perception of food, including otherwise unpalatable food. Salting, brining, and pickling are also ancient and important methods of food preservation. Some of the earliest evidence of salt processing dates to around 6,000 BC, when people living in the area of present-day Romania boiled spring water to extract salts; a salt-works in China dates to approximately the same period. Salt was also prized by the ancient Hebrews, Greeks, Romans, Byzantines, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |