Weinreb Ketone Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Weinreb–Nahm ketone synthesis is a chemical reaction used in  The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents of the incoming group add to form an
The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents of the incoming group add to form an  The Weinreb–Nahm amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These
The Weinreb–Nahm amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These
 This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
 A variety of
A variety of  Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of
Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of 
 Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the
Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the 
 Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
 More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone
More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone 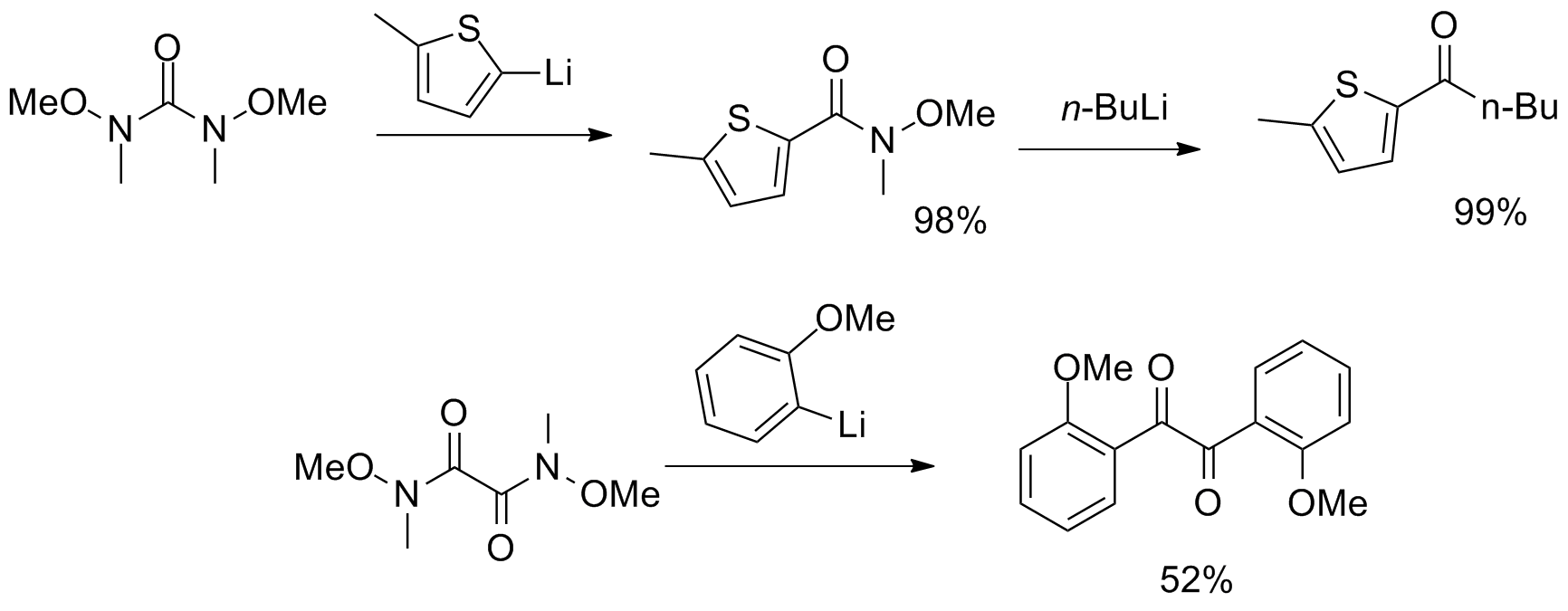 Finally, Stephen G. Davies of
Finally, Stephen G. Davies of 
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
to make carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed b ...
s. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
. The original reaction involved two subsequent nucleophilic acyl substitution
Nucleophilic acyl substitution describe a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl deriv ...
s: the conversion of an acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
with N,O-Dimethylhydroxylamine
''N'',''O''-Dimethylhydroxylamine is a methylated hydroxylamine used to form so called 'Weinreb amides' for use in the Weinreb ketone synthesis. It is commercially available as its hydrochloride salt.
Synthesis
It may be prepared by reacting e ...
, to form a Weinreb–Nahm amide, and subsequent treatment of this species with an organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
reagent such as a Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
or organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
. Nahm and Weinreb also reported the synthesis of aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s by reduction of the amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
with an excess of lithium aluminum hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic sy ...
(see amide reduction Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.
Catalytic hydrogenation
Catalytic hydrogenation can be used to reduce amides to amines; however, the process often req ...
).
 The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents of the incoming group add to form an
The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents of the incoming group add to form an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
rather than a ketone or aldehyde. This occurs even if the equivalents of nucleophile are closely controlled.
 The Weinreb–Nahm amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These
The Weinreb–Nahm amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s are present in a large number of natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
s and can be reliably reacted to form new carbon–carbon bonds or converted into other functional groups. This method has been used in a number of syntheses, including macrosphelides A and B, amphidinolide J, and spirofungins A and B. (See Scope
Scope or scopes may refer to:
People with the surname
* Jamie Scope (born 1986), English footballer
* John T. Scopes (1900–1970), central figure in the Scopes Trial regarding the teaching of evolution
Arts, media, and entertainment
* Cinem ...
below)
Mechanism
Weinreb and Nahm originally proposed the followingreaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
to explain the selectivity shown in reactions of the Weinreb–Nahm amide. Their suggestion was that the tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
(A below) formed as a result of nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
by the organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
reagent is stabilized by chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a Denticity, polydentate (multiple bonded) ligand and a single central metal atom. These l ...
from the methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as ...
group as shown. This intermediate is stable only at low temperatures, requiring a low-temperature quench
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phas ...
.
 This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
Preparation
In addition to the original procedure shown above (which may have compatibility issues for sensitive substrates), Weinreb amides can be synthesized from a variety ofacyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC n ...
compounds. The vast majority of these procedures utilize the commercially available salt N,O-dimethylhydroxylamine hydrochloride eO(Me)NH•HCl which is typically easier to handle than the free amine.
Treatment of an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
or lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
with AlMe3 or AlMe2Cl affords the corresponding Weinreb amide in good yields. Alternatively, non-nucleophilic Grignard reagents such as isopropyl magnesium chloride can be used to activate the amine before addition of the ester.
 A variety of
A variety of peptide coupling
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl ...
reagents can also be used to prepare Weinreb–Nahm amides from carboxylic acids. Various carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. ...
-, hydroxybenzotriazole
Hydroxybenzotriazole (abbreviated HOBt) is an organic compound that is a derivative of benzotriazole. It is a white crystalline powder, which as a commercial product contains some water (~11.7% wt as the HOBt monohydrate crystal). Anhydrous HOBt ...
-, and triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists a ...
-based couplings have been reported specifically for this purpose.
 Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of
Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
halides directly into aryl Weinreb–Nahm amides.

Scope
The standard conditions for the Weinreb–Nahm ketone synthesis are known to tolerate a wide variety of functional groups elsewhere in the molecule, including alpha-halogen substitution, N-protectedamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s, α-β unsaturation, silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting g ...
s, various lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lacta ...
and lactones, sulfonates
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-ox ...
, sulfinates, and phosphonate esters. A wide variety of nucleophiles can be used in conjunction with the amide. Lithiates and Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
s are most commonly employed; examples involving aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
, vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
, and alkynyl
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
carbon nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s have been reported. However, with highly basic or sterically hindered nucleophiles, elimination of the methoxide moiety to release formaldehyde can occur as a significant side reaction.
 Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the
Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the immunosuppressant
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent activity of the immune system.
Classification
Immunosuppressive drugs can be classified in ...
family of macrosphelides, and the antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
family of spirofungins.

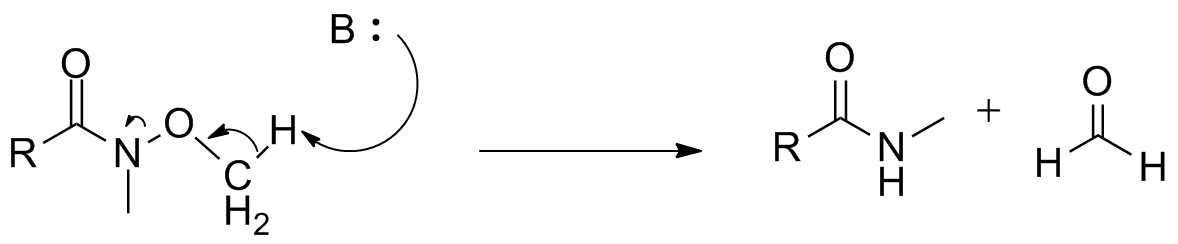
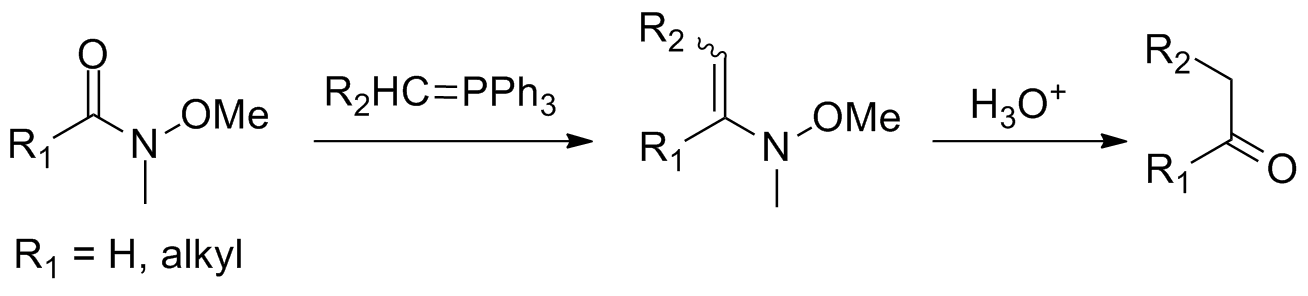
Variations
Reaction of Weinreb–Nahm amides withWittig reagents In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes:
:
Preparation
Because they typically hydrolyze and oxidize readily ...
has been performed to avoid the sometimes harsh conditions required for addition of hydride reagents or organometallic compounds. This yields an N-methyl-N-methoxy-enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and th ...
that converts to the corresponding ketone or aldehyde upon hydrolytic workup.
 Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
 More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone
More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 19 ...
s.
 Finally, Stephen G. Davies of
Finally, Stephen G. Davies of Oxford
Oxford () is a city in England. It is the county town and only city of Oxfordshire. In 2020, its population was estimated at 151,584. It is north-west of London, south-east of Birmingham and north-east of Bristol. The city is home to the ...
has designed a chiral auxiliary
In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the ...
that combines the functionality of the Weinreb amide with that of the Myers' pseudoephedrine
Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.
It was first characteri ...
auxiliary, allowing diastereoselective enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
alkylation followed by facile cleavage to the corresponding enantioenriched aldehyde or ketone.

See also
*N,O-Dimethylhydroxylamine
''N'',''O''-Dimethylhydroxylamine is a methylated hydroxylamine used to form so called 'Weinreb amides' for use in the Weinreb ketone synthesis. It is commercially available as its hydrochloride salt.
Synthesis
It may be prepared by reacting e ...
* Ketone#Synthesis
References
{{DEFAULTSORT:Weinreb Ketone Synthesis Carbon-carbon bond forming reactions Substitution reactions Name reactions