|
Lithium Aluminium Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Storage
Hydrogen storage can be accomplished by several existing methods of holding hydrogen for later use. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. Interest in using hydrogen for on-board storage of energy in zero-emissions vehicles is motivating the development of new methods of storage, more adapted to this new application. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy. E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lialh4 Xrpd
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ... Lialuminium, Alhydride, H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bipyramid
A (symmetric) -gonal bipyramid or dipyramid is a polyhedron formed by joining an -gonal pyramid and its mirror image base-to-base. An -gonal bipyramid has triangle faces, edges, and vertices. The "-gonal" in the name of a bipyramid does not refer to a face but to the internal polygon base, lying in the mirror plane that connects the two pyramid halves. (If it were a face, then each of its edges would connect three faces instead of two.) "Regular", right bipyramids A ''"regular"'' bipyramid has a ''regular'' polygon base. It is usually implied to be also a ''right'' bipyramid. A ''right'' bipyramid has its two apices ''right'' above and ''right'' below the center or the '' centroid'' of its polygon base. A "regular" right (symmetric) -gonal bipyramid has Schläfli symbol . A right (symmetric) bipyramid has Schläfli symbol , for polygon base . The "regular" right (thus face-transitive) -gonal bipyramid with regular vertices is the dual of the -gonal uniform (thus ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ordinary convex polyhedra and the only one that has fewer than 5 faces. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such nets. For any tetrahedron there exists a sphere (called the circumsphere) on which all four vertices lie, and anot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
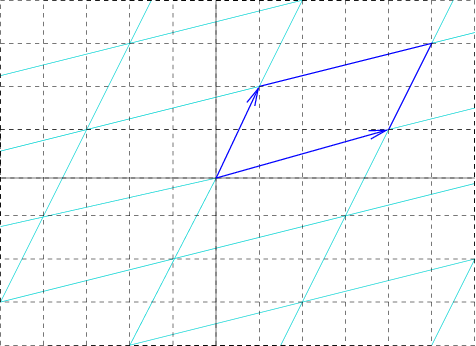
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Hydroxide
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina (Al2O3), the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water. Structure Al(OH)3 is built up of double layers of hydroxyl groups with aluminium ions occupying two-thirds of the octahedral holes between the two layers. Four polymorphs are recognized. All feature layers of octahedral aluminium hydroxide units, with hydrogen bonds between the layers. The polymorphs differ in terms of the stacking of the layers. All forms of Al(OH)3 crystals are hexagonal : *gibbsite is also known as γ-Al(OH)3 or α-Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids. They are soluble in water and slightly soluble in ethanol. Both are available commercially. While classified as a strong base, lithium hydroxide is the weakest known alkali metal hydroxide. Production The preferred feedstock is hard-rock spodumene, where the lithium content is expressed as % lithium oxide. Lithium carbonate route Lithium hydroxide is often produced industrially from lithium carbonate in a metathesis reaction with calcium hydroxide: : The initially produced hydrate is dehydrated by heating under vacuum up to 180 °C. Lithium sulfate route An alternative route involves the intermediacy of lithium sulfate: :α-spodumene → β-spodumene :β-spodumene + CaO → + ... : : The main by-products are gypsum and sodium sulphate, which have some market value. Commercial setting According to Bloomberg, Ganfeng Lit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral Oil
Mineral oil is any of various colorless, odorless, light mixtures of higher alkanes from a mineral source, particularly a distillate of petroleum, as distinct from usually edible vegetable oils. The name 'mineral oil' by itself is imprecise, having been used for many specific oils over the past few centuries. Other names, similarly imprecise, include 'white oil', 'paraffin oil', ' liquid paraffin' (a highly refined medical grade), (Latin), and 'liquid petroleum'. Most often, mineral oil is a liquid by-product of refining crude oil to make gasoline and other petroleum products. This type of mineral oil is a transparent, colorless oil, composed mainly of alkanes and cycloalkanes, related to petroleum jelly. It has a density of around . Nomenclature Some of the imprecision in the definition of the names used for mineral oil (such as 'white oil') reflects usage by consumers and merchants who did not know, and usually had no need of knowing, the oil's precise chemical mak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or (with a few exceptions) nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed. Uses The creation of sparks from metals is based on the pyrophoricity of small metal particles, and pyrophoric alloys are made for this purpose. The sparking mechanisms in lighters and various toys, using ferrocerium; starting fires without matches, using a firesteel; the flintlock mechanism in firearms; and spark testing ferr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)