Water () is a
polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
that is at
room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
a tasteless and odorless
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
, which is nearly colorless apart from
an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
" and the "solvent of life". It is the most abundant substance on the surface of
Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surfa ...
and the only common substance to exist as a
solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
, liquid, and
gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
on Earth's surface. It is also the third most abundant molecule in the universe (behind
molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, ...
and
carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
).
Water molecules form
hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate
ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high
boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
of 100 °C for its
molar mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, p ...
, and a high
heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity i ...
.
Water is
amphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
One type of amphoteric species are amphipro ...
, meaning that it can exhibit properties of an
acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
or a
base, depending on the pH of the solution that it is in; it readily produces both
and
ions. Related to its amphoteric character, it undergoes
self-ionization. The product of the
activities, or approximately, the concentrations of and is a constant, so their respective concentrations are inversely proportional to each other.
Physical properties
Water is the
chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
with
chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
; one
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
of water has two
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s
covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
ly
bonded to a single
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
atom. Water is a tasteless, odorless liquid at
ambient temperature and pressure. Liquid water has weak
absorption band
According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When such quanta of electromagnetic radiation are emitted or absorbed by an atom or molecule, energy of the ...
s at wavelengths of around 750 nm which cause it to appear to have a blue colour.
This can easily be observed in a water-filled bath or wash-basin whose lining is white. Large ice crystals, as in
glacier
A glacier (; ) is a persistent body of dense ice that is constantly moving under its own weight. A glacier forms where the accumulation of snow exceeds its Ablation#Glaciology, ablation over many years, often Century, centuries. It acquires dis ...
s, also appear blue.
Under
standard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
, water is primarily a liquid, unlike other analogous hydrides of the
oxygen family, which are generally gaseous. This unique property of water is due to
hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
. The molecules of water are constantly moving concerning each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (2 × 10
−13 seconds). However, these bonds are strong enough to create many of the peculiar properties of water, some of which make it integral to life.
Water, ice, and vapour
Within the Earth's atmosphere and surface, the
liquid phase
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, an ...
is the most common and is the form that is generally denoted by the word "water". The
solid phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetizati ...
of water is known as
ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaq ...
and commonly takes the structure of hard, amalgamated
crystals
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
, such as
ice cubes
An ice cube is a small piece of ice, which is typically rectangular as viewed from above and trapezoidal as viewed from the side. Ice cubes are products of mechanical refrigeration and are usually produced to cool beverages. They may be produc ...
, or loosely accumulated
granular
Granularity (also called graininess), the condition of existing in granules or grains, refers to the extent to which a material or system is composed of distinguishable pieces. It can either refer to the extent to which a larger entity is subd ...
crystals, like
snow
Snow comprises individual ice crystals that grow while suspended in the atmosphere—usually within clouds—and then fall, accumulating on the ground where they undergo further changes.
It consists of frozen crystalline water throughout ...
. Aside from
common hexagonal crystalline ice, other crystalline and amorphous
phases of ice are known. The
gaseous phase
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
of water is known as
water vapor
(99.9839 °C)
, -
, Boiling point
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous pha ...
(or
steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
). Visible steam and clouds are formed from minute droplets of water suspended in the air.
Water also forms a
supercritical fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous so ...
. The
critical temperature
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
*Critical juncture, a discontinuous change studied in the social sciences.
*Critical Software, a company specializing in ...
is 647
K and the
critical pressure
In thermodynamics, a critical point (or critical state) is the end point of a phase equilibrium curve. The most prominent example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions ...
is 22.064
MPa
MPA or mPa may refer to:
Academia
Academic degrees
* Master of Performing Arts
* Master of Professional Accountancy
* Master of Public Administration
* Master of Public Affairs
Schools
* Mesa Preparatory Academy
* Morgan Park Academy
* Mound ...
. In nature, this only rarely occurs in extremely hostile conditions. A likely example of naturally occurring supercritical water is in the hottest parts of deep water
hydrothermal vents
A hydrothermal vent is a fissure on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspot ...
, in which water is heated to the critical temperature by
volcanic
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plates a ...
plumes and the critical pressure is caused by the weight of the ocean at the extreme depths where the vents are located. This pressure is reached at a depth of about 2200 meters: much less than the mean depth of the ocean (3800 meters).
Heat capacity and heats of vaporization and fusion
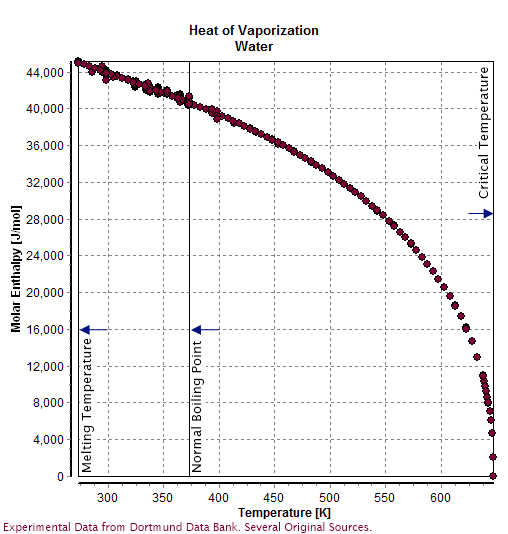
Water has a very high
specific heat capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat t ...
of 4184 J/(kg·K) at 20 °C (4182 J/(kg·K) at 25 °C) —the second-highest among all the heteroatomic species (after
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
), as well as a high
heat of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
(40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing between its molecules. These two unusual properties allow water to moderate Earth's
climate
Climate is the long-term weather pattern in an area, typically averaged over 30 years. More rigorously, it is the mean and variability of meteorological variables over a time spanning from months to millions of years. Some of the meteorologic ...
by buffering large fluctuations in temperature. Most of the additional energy stored in the climate system since 1970 has accumulated in the oceans.
The specific
enthalpy of fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a s ...
(more commonly known as latent heat) of water is 333.55 kJ/kg at 0 °C: the same amount of energy is required to melt ice as to warm ice from −160 °C up to its melting point or to heat the same amount of water by about 80 °C. Of common substances, only that of ammonia is higher. This property confers resistance to melting on the ice of
glacier
A glacier (; ) is a persistent body of dense ice that is constantly moving under its own weight. A glacier forms where the accumulation of snow exceeds its Ablation#Glaciology, ablation over many years, often Century, centuries. It acquires dis ...
s and
drift ice
Drift ice, also called brash ice, is sea ice that is not attached to the shoreline or any other fixed object (shoals, grounded icebergs, etc.).Leppäranta, M. 2011. The Drift of Sea Ice. Berlin: Springer-Verlag. Unlike fast ice, which is "fasten ...
. Before and since the advent of mechanical
refrigeration
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, ht ...
, ice was and still is in common use for retarding food spoilage.
The specific heat capacity of ice at −10 °C is 2030 J/(kg·K) and the heat capacity of steam at 100 °C is 2080 J/(kg·K).
Density of water and ice

The
density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of water is about : this relationship was originally used to define the gram.
The density varies with temperature, but not linearly: as the temperature increases, the density rises to a peak at and then decreases; the initial increase is unusual because most liquids undergo
thermal expansion
Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions.
Temperature is a monotonic function of the average molecular kinetic ...
so that the density only decreases as a function of temperature. The increase observed for water from to and for a few other liquids is described as
negative thermal expansion. Regular,
hexagonal ice is also less dense than liquid water—upon freezing, the density of water decreases by about 9%.
These peculiar effects are due to the highly directional bonding of water molecules via the hydrogen bonds: ice and liquid water at low temperature have comparatively low-density, low-energy open lattice structures. The breaking of hydrogen bonds on melting with increasing temperature in the range 0–4 °C allows for a denser molecular packing in which some of the lattice cavities are filled by water molecules.
Above 4 °C, however, thermal expansion becomes the dominant effect,
[ and water near the boiling point (100 °C) is about 4% less dense than water at .]ice II
Ice II is a rhombohedral crystalline form of ice with a highly ordered structure. It is formed from ice Ih by compressing it at a temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III. Ordi ...
, ice III
Ice III is a form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to at . It is the least dense of the high-pressure water phases, with a density of (at 350 MPa). It has a very high relative permittiv ...
, high-density amorphous ice
Amorphous ice (non-crystalline or "vitreous" ice) is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice has a lack of long-range order ...
(HDA), and very-high-density amorphous ice
Amorphous ice (non-crystalline or "vitreous" ice) is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice has a lack of long-range order ...
(VHDA).
 The unusual density curve and lower density of ice than of water is essential for much of the life on earth—if water were most dense at the freezing point, then in winter the cooling at the surface would lead to convective mixing. Once 0 °C are reached, the water body would freeze from the bottom up, and all life in it would be killed.
The unusual density curve and lower density of ice than of water is essential for much of the life on earth—if water were most dense at the freezing point, then in winter the cooling at the surface would lead to convective mixing. Once 0 °C are reached, the water body would freeze from the bottom up, and all life in it would be killed.Lake Baikal
Lake Baikal (, russian: Oзеро Байкал, Ozero Baykal ); mn, Байгал нуур, Baigal nuur) is a rift lake in Russia. It is situated in southern Siberia, between the federal subjects of Irkutsk Oblast to the northwest and the Repu ...
in central Siberia
Siberia ( ; rus, Сибирь, r=Sibir', p=sʲɪˈbʲirʲ, a=Ru-Сибирь.ogg) is an extensive geographical region, constituting all of North Asia, from the Ural Mountains in the west to the Pacific Ocean in the east. It has been a part of ...
freezes only to about 1 m thickness in winter. In general, for deep enough lakes, the temperature at the bottom stays constant at about 4 °C (39 °F) throughout the year (see diagram).
Density of saltwater and ice
 The density of saltwater depends on the dissolved salt content as well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 1.9 °C
The density of saltwater depends on the dissolved salt content as well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 1.9 °Chere
Here is an adverb that means "in, on, or at this place". It may also refer to:
Software
* Here Technologies, a mapping company
* Here WeGo (formerly Here Maps), a mobile app and map website by Here
Television
* Here TV (formerly "here!"), a TV ...
for explanation) and lowers the temperature of the density maximum of water to the former freezing point at 0 °C. This is why, in ocean water, the downward convection of colder water is ''not'' blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. So creatures that live at the bottom of cold oceans like the Arctic Ocean
The Arctic Ocean is the smallest and shallowest of the world's five major oceans. It spans an area of approximately and is known as the coldest of all the oceans. The International Hydrographic Organization (IHO) recognizes it as an ocean, a ...
generally live in water 4 °C colder than at the bottom of frozen-over fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does include ...
lakes and rivers.
As the surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is t ...
of saltwater begins to freeze (at −1.9 °Cseawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
, 3.5%) the ice that forms is essentially salt-free, with about the same density as freshwater ice. This ice floats on the surface, and the salt that is "frozen out" adds to the salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
and density of the seawater just below it, in a process known as ''brine rejection
Brine rejection is a process that occurs when salty water freezes. The salts do not fit in the crystal structure of water ice, so the salt is expelled.
Since the oceans are salty, this process is important in nature. Salt rejected by the forming ...
''. This denser saltwater sinks by convection and the replacing seawater is subject to the same process. This produces essentially freshwater ice at −1.9 °Cthermohaline circulation
Thermohaline circulation (THC) is a part of the large-scale ocean circulation that is driven by global density gradients created by surface heat and freshwater fluxes. The adjective ''thermohaline'' derives from '' thermo-'' referring to temper ...
.
Miscibility and condensation
 Water is
Water is miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies ...
with many liquids, including ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
in all proportions. Water and most oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturated ...
s are immiscible usually forming layers according to increasing density from the top. This can be predicted by comparing the polarity
Polarity may refer to:
Science
*Electrical polarity, direction of electrical current
*Polarity (mutual inductance), the relationship between components such as transformer windings
* Polarity (projective geometry), in mathematics, a duality of ord ...
. Water being a relatively polar compound will tend to be miscible with liquids of high polarity such as ethanol and acetone, whereas compounds with low polarity will tend to be immiscible and poorly soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
such as with hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
.
As a gas, water vapor is completely miscible with air. On the other hand, the maximum water vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor's partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas ...
is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C, water will start to condense, defining the dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
, and creating fog
Fog is a visible aerosol consisting of tiny water droplets or ice crystals suspended in the air at or near the Earth's surface. Reprint from Fog can be considered a type of low-lying cloud usually resembling stratus, and is heavily influ ...
or dew
Dew is water in the form of droplets that appears on thin, exposed objects in the morning or evening due to condensation.
As the exposed surface cools by radiating its heat, atmospheric moisture condenses at a rate greater than that at wh ...
. The reverse process accounts for the fog burning off in the morning. If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change and then condenses out as minute water droplets, commonly referred to as steam.
A saturated gas or one with 100% relative humidity is when the vapor pressure of water in the air is at equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in the air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. Vapor pressure above 100% relative humidity is called supersaturated and can occur if the air is rapidly cooled, for example, by rising suddenly in an updraft.
Vapor pressure

Compressibility
The compressibility
In thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility or, if the temperature is held constant, the isothermal compressibility) is a measure of the instantaneous relative volume change of a fl ...
of water is a function of pressure and temperature. At 0 °C, at the limit of zero pressure, the compressibility is . At the zero-pressure limit, the compressibility reaches a minimum of around 45 °C before increasing again with increasing temperature. As the pressure is increased, the compressibility decreases, being at 0 °C and .
The bulk modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other moduli describe ...
of water is about 2.2 GPa.ocean
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth and contains 97% of Earth's water. An ocean can also refer to any of the large bodies of water into which the wo ...
s at 4 km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.[
The bulk modulus of water ice ranges from 11.3 GPa at 0 K up to 8.6 GPa at 273 K. The large change in the compressibility of ice as a function of temperature is the result of its relatively large thermal expansion coefficient compared to other common solids.
]
Triple point
 The
The temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
at which ordinary solid, liquid, and gaseous water coexist in equilibrium is a triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the subli ...
of water. Since 1954, this point had been used to define the base unit of temperature, the kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phys ...
, but, starting in 2019, the kelvin is now defined using the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
, rather than the triple point of water.
Due to the existence of many polymorphs (forms) of ice, water has other triple points, which have either three polymorphs of ice or two polymorphs of ice and liquid in equilibrium. Gustav Heinrich Johann Apollon Tammann
Gustav Heinrich Johann Apollon Tammann ( – 17 December 1938) was a prominent Baltic German chemist-physicist who made important contributions in the fields of glassy and solid solutions, heterogeneous equilibria, crystallization, and metallurg ...
in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.
Melting point
The melting point of ice is at standard pressure; however, pure liquid water can be supercooled well below that temperature without freezing if the liquid is not mechanically disturbed. It can remain in a fluid state down to its homogeneous nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that deter ...
point of about . The melting point of ordinary hexagonal ice falls slightly under moderately high pressures, by /atm or about /70 atm as the stabilization energy of hydrogen bonding is exceeded by intermolecular repulsion, but as ice transforms into its polymorphs (see crystalline states of ice) above , the melting point increases markedly with pressure, i.e., reaching at (triple point of Ice VII
Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, fr ...
Electrical properties
Electrical conductivity
Pure water containing no exogenous ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s is an excellent electronic insulator, but not even "deionized" water is completely free of ions. Water undergoes autoionization
Autoionization is a process by which an atom or a molecule in an excited state spontaneously emits one of the outer-shell electrons, thus going from a state with charge to a state with charge , for example from an electrically neutral st ...
in the liquid state when two water molecules form one hydroxide anion () and one hydronium cation (). Because of autoionization, at ambient temperatures pure liquid water has a similar intrinsic charge carrier concentration to the semiconductor germanium and an intrinsic charge carrier concentration three orders of magnitude greater than the semiconductor silicon, hence, based on charge carrier concentration, water can not be considered to be a completely dielectric material or electrical insulator but to be a limited conductor of ionic charge.
Because water is such a good solvent, it almost always has some solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent ...
dissolved in it, often a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
. If water has even a tiny amount of such an impurity, then the ions can carry charges back and forth, allowing the water to conduct electricity far more readily.
It is known that the theoretical maximum electrical resistivity for water is approximately 18.2 MΩ·cm (182 kΩ·m) at 25 °C.[ This figure agrees well with what is typically seen on ]reverse osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pre ...
, ultra-filtered and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.
In pure water, sensitive equipment can detect a very slight electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
of 0.05501 ± 0.0001 μS/ cm at 25.00 °C.protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
(see proton conductor
A proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers.
Composition
Acid solutions exhibit proton-conductivity, while pure proton conductors are usually dry solids. Typical materials a ...
). Ice was previously thought to have a small but measurable conductivity of 1 S/cm, but this conductivity is now thought to be almost entirely from surface defects, and without those, ice is an insulator with an immeasurably small conductivity.
Polarity and hydrogen bonding
 An important feature of water is its polar nature. The structure has a
An important feature of water is its polar nature. The structure has a bent molecular geometry
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-colline ...
for the two hydrogens from the oxygen vertex. The oxygen atom also has two lone pairs
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons. One effect usually ascribed to the lone pairs is that the H–O–H gas-phase bend angle is 104.48°, which is smaller than the typical tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
angle of 109.47°. The lone pairs are closer to the oxygen atom than the electrons sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
ed to the hydrogens, so they require more space. The increased repulsion of the lone pairs forces the O–H bonds closer to each other.
Another consequence of its structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
is that water is a polar molecule
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
. Due to the difference in electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
, a bond dipole moment
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
points from each H to the O, making the oxygen partially negative and each hydrogen partially positive. A large molecular dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
, points from a region between the two hydrogen atoms to the oxygen atom. The charge differences cause water molecules to aggregate (the relatively positive areas being attracted to the relatively negative areas). This attraction, hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing, explains many of the properties of water, such as its solvent properties.
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for several of the water's physical properties. These properties include its relatively high melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which incre ...
and boiling point temperatures: more energy is required to break the hydrogen bonds between water molecules. In contrast, hydrogen sulfide (), has much weaker hydrogen bonding due to sulfur's lower electronegativity. is a gas at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, despite hydrogen sulfide having nearly twice the molar mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat t ...
. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
Cohesion and adhesion
 Water molecules stay close to each other ( cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high
Water molecules stay close to each other ( cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
The forces that cause adhesion and cohesion can be ...
properties because of its polar nature. On clean, smooth glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of ...
the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces. In biological cells and organelle
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as organs are to the body, hence ''organelle,'' the ...
s, water is in contact with membrane and protein surfaces that are hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
; that is, surfaces that have a strong attraction to water. Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publication is the 1919 art ...
observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less. They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.

Surface tension
 Water has an unusually high
Water has an unusually high surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
of 71.99 mN/m at 25 °C which is caused by the strength of the hydrogen bonding between water molecules. This allows insects to walk on water.
Capillary action
Because water has strong cohesive and adhesive forces, it exhibits capillary action. Strong cohesion from hydrogen bonding and adhesion allows trees to transport water more than 100 m upward.
Water as a solvent
 Water is an excellent
Water is an excellent solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
due to its high dielectric constant. Substances that mix well and dissolve in water are known as hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
("water-loving") substances, while those that do not mix well with water are known as hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
("water-fearing") substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are precipitated out from the water. Contrary to the common misconception, water and hydrophobic substances do not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
When an ionic or polar compound enters water, it is surrounded by water molecules (hydration Hydration may refer to:
* Hydrate, a substance that contains water
* Hydration enthalpy, energy released through hydrating a substance
* Hydration reaction, a chemical addition reaction where a hydroxyl group and proton are added to a compound
* ...
). The relatively small size of water molecules (~ 3 angstroms) allows many water molecules to surround one molecule of solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent ...
. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s, alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s, and salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
s are relatively soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with non-polar molecules.
An example of an ionic solute is table salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
; the sodium chloride, NaCl, separates into cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s and anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
into solution. An example of a nonionic solute is table sugar
White sugar, also called table sugar, granulated sugar, or regular sugar, is a commonly used type of sugar, made either of beet sugar or cane sugar, which has undergone a refining process.
Description
The refining process completely removes ...
. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
Quantum tunneling
The quantum tunneling
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantizati ...
dynamics in water was reported as early as 1992. At that time it was known that there are motions which destroy and regenerate the weak hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
by internal rotations of the substituent water monomers
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
M ...
. On 18 March 2016, it was reported that the hydrogen bond can be broken by quantum tunneling in the water hexamer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative ...
. Unlike previously reported tunneling motions in water, this involved the concerted breaking of two hydrogen bonds. Later in the same year, the discovery of the quantum tunneling of water molecules was reported.
Electromagnetic absorption
Water is relatively transparent to visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
, near ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
light, and far-red light, but it absorbs most ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
, infrared light
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
, and microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ran ...
s. Most photoreceptors and photosynthetic pigment
A photosynthetic pigment (accessory pigment; chloroplast pigment; antenna pigment) is a pigment that is present in chloroplasts or photosynthetic bacteria and captures the light energy necessary for photosynthesis.
List of photosynthetic pigmen ...
s utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens
A microwave oven (commonly referred to as a microwave) is an electric oven that heats and cooks food by exposing it to electromagnetic radiation in the microwave frequency range. This induces polar molecules in the food to rotate and produce th ...
take advantage of water's opacity to microwave radiation to heat the water inside of foods. Water's light blue colour is caused by weak absorption
Absorption may refer to:
Chemistry and biology
* Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which ...
in the red part of the visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' or simply light. A typical human eye wil ...
.
Structure
 A single water molecule can participate in a maximum of four
A single water molecule can participate in a maximum of four hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s because it can accept two bonds using the lone pairs on oxygen and donate two hydrogen atoms. Other molecules like hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
, ammonia, and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
can also form hydrogen bonds. However, they do not show anomalous thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ther ...
, kinetic
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory of gases, Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to i ...
, or structural properties like those observed in water because none of them can form four hydrogen bonds: either they cannot donate or accept hydrogen atoms, or there are steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
effects in bulky residues. In water, intermolecular tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
structures form due to the four hydrogen bonds, thereby forming an open structure and a three-dimensional bonding network, resulting in the anomalous decrease in density when cooled below 4 °C. This repeated, constantly reorganizing unit defines a three-dimensional network extending throughout the liquid. This view is based upon neutron scattering studies and computer simulations, and it makes sense in the light of the unambiguously tetrahedral arrangement of water molecules in ice structures.
However, there is an alternative theory for the structure of water. In 2004, a controversial paper from Stockholm University
Stockholm University ( sv, Stockholms universitet) is a public research university in Stockholm, Sweden, founded as a college in 1878, with university status since 1960. With over 33,000 students at four different faculties: law, humanities, so ...
suggested that water molecules in the liquid state typically bind not to four but only two others; thus forming chains and rings. The term "string theory of water" (which is not to be confused with the string theory
In physics, string theory is a theoretical framework in which the point-like particles of particle physics are replaced by one-dimensional objects called strings. String theory describes how these strings propagate through space and interac ...
of physics) was coined. These observations were based upon X-ray absorption spectroscopy that probed the local environment of individual oxygen atoms.
Molecular structure
The repulsive effects of the two lone pairs on the oxygen atom cause water to have a bent, not linear
Linearity is the property of a mathematical relationship (''function'') that can be graphically represented as a straight line. Linearity is closely related to '' proportionality''. Examples in physics include rectilinear motion, the linear r ...
, molecular structure, allowing it to be polar. The hydrogen–oxygen–hydrogen angle is 104.45°, which is less than the 109.47° for ideal sp3 hybridization. The valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
explanation is that the oxygen atom's lone pairs are physically larger and therefore take up more space than the oxygen atom's bonds to the hydrogen atoms. The molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
explanation (Bent's rule
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization of central atoms in molecules and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows:
The chemical structu ...
) is that lowering the energy of the oxygen atom's nonbonding hybrid orbitals (by assigning them more s character and less p character) and correspondingly raising the energy of the oxygen atom's hybrid orbitals bonded to the hydrogen atoms (by assigning them more p character and less s character) has the net effect of lowering the energy of the occupied molecular orbitals because the energy of the oxygen atom's nonbonding hybrid orbitals contributes completely to the energy of the oxygen atom's lone pairs while the energy of the oxygen atom's other two hybrid orbitals contributes only partially to the energy of the bonding orbitals (the remainder of the contribution coming from the hydrogen atoms' 1s orbitals).
Chemical properties
Self-ionization
In liquid water there is some self-ionization giving hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is d ...
ions and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ions.
:2 +
The equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for this reaction, known as the ionic product of water, , has a value of about at 25 °C. At neutral pH, the concentration of the hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ion () equals that of the (solvated) hydrogen ion (), with a value close to 10−7 mol L−1 at 25 °C. See data page for values at other temperatures.
The thermodynamic equilibrium constant is a quotient of thermodynamic activities of all products and reactants including water:
:
However for dilute solutions, the activity of a solute such as H3O+ or OH− is approximated by its concentration, and the activity of the solvent H2O is approximated by 1, so that we obtain the simple ionic product
Geochemistry
The action of water on rock over long periods of time typically leads to weathering and water erosion, physical processes that convert solid rocks and minerals into soil and sediment, but under some conditions chemical reactions with water occur as well, resulting in metasomatism
Metasomatism (from the Greek μετά ''metá'' "change" and σῶμα ''sôma'' "body") is the chemical alteration of a rock by hydrothermal and other fluids. It is the replacement of one rock by another of different mineralogical and chemical com ...
or mineral hydration
In chemistry, mineral hydration is an inorganic chemical reaction which adds water to the crystal structure of a mineral, usually creating a new mineral, usually called a ''hydrate''.
In geological terms, the process of mineral hydration is know ...
, a type of chemical alteration of a rock which produces clay minerals
Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2 Si2 O5( OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces.
Clay mineral ...
. It also occurs when Portland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in the early 19th c ...
hardens.
Water ice can form clathrate compounds
A clathrate is a chemical substance consisting of a lattice (group), lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin language, Latin (), meaning ‘with bars, Crystal structure, latticed’. Most clathr ...
, known as clathrate hydrates
Clathrate hydrates, or gas hydrates, clathrates, hydrates, etc., are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
, with a variety of small molecules that can be embedded in its spacious crystal lattice. The most notable of these is methane clathrate
Methane clathrate (CH4·5.75H2O) or (8CH4·46H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amou ...
, 4 , naturally found in large quantities on the ocean floor.
Acidity in nature
Rain is generally mildly acidic, with a pH between 5.2 and 5.8 if not having any acid stronger than carbon dioxide. If high amounts of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
oxides are present in the air, they too will dissolve into the cloud and raindrops, producing acid rain
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid ...
.
Isotopologues
Several isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s of both hydrogen and oxygen exist, giving rise to several known isotopologue
In chemistry, isotopologues are molecules that differ only in their isotopic composition. They have the same chemical formula and bonding arrangement of atoms, but at least one atom has a different number of neutrons than the parent.
An exampl ...
s of water. Vienna Standard Mean Ocean Water
Vienna Standard Mean Ocean Water (VSMOW) is an isotopic standard for water. Despite the name, VSMOW is pure water with no salt or other chemicals found in the oceans. The VSMOW standard was promulgated by the International Atomic Energy Agency ( ...
is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope protium. Only 155 ppm include deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium ato ...
( or D), a hydrogen isotope with one neutron, and fewer than 20 parts per quintillion
Two naming scales for large numbers have been used in English and other European languages since the early modern era: the long and short scales. Most English variants use the short scale today, but the long scale remains dominant in many non-Eng ...
include tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus o ...
( or T), which has two neutrons. Oxygen also has three stable isotopes, with present in 99.76%, in 0.04%, and in 0.2% of water molecules.
Deuterium oxide, , is also known as heavy water because of its higher density. It is used in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely mo ...
. Tritium is radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
, decaying with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of 4500 days; exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one protium and one deuterium atom occur naturally in ordinary water in low concentrations (~0.03%) and in far lower amounts (0.000003%) and any such molecules are temporary as the atoms recombine.
The most notable physical differences between and , other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. The difference in boiling points allows the isotopologues to be separated. The self-diffusion According to IUPAC definition, self-diffusion coefficient is the diffusion coefficient D_i^* of species i when the chemical potential gradient equals zero. It is linked to the diffusion coefficient D_i by the equation:
D_i^*=D_i\frac.
Here, a_i is ...
coefficient of at 25 °C is 23% higher than the value of .
Occurrence
Water is the most abundant substance on Earth and also the third most abundant molecule in the universe, after and . 0.23 ppm of the earth's mass is water and 97.39% of the global water volume of 1.38 km3 is found in the oceans.
Reactions
Acid-base reactions
Water is amphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
One type of amphoteric species are amphipro ...
: it has the ability to act as either an acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
or a base in chemical reactions. According to the Brønsted-Lowry definition, an acid is a proton () donor and a base is a proton acceptor. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, water receives an ion from HCl when hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
is formed:
: + +
In the reaction with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
, , water donates a ion, and is thus acting as an acid:
: + +
Because the oxygen atom in water has two lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s, water often acts as a Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, or electron-pair donor, in reactions with Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory
HSAB concept is a jargon for "hard and soft Lewis acids and bases, (Lewis) acids and bases". HSAB is widely used in chemistry for explaining stability of chemical compound, compounds, reaction mechanisms and pathways. It assigns the terms 'hard' o ...
describes water as both a weak hard acid and a weak hard base, meaning that it reacts preferentially with other hard species:
: + →
: + →
: + →
When a salt of a weak acid or of a weak base is dissolved in water, water can partially hydrolyze
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
the salt, producing the corresponding base or acid, which gives aqueous solutions of soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are use ...
and baking soda
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
their basic pH:
: + NaOH +
Ligand chemistry
Water's Lewis base character makes it a common ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
in transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
complexes, examples of which include metal aquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
es such as to perrhenic acid
Perrhenic acid is the chemical compound with the formula . It is obtained by evaporating aqueous solutions of . Conventionally, perrhenic acid is considered to have the formula , and a species of this formula forms when rhenium(VII) oxide sublime ...
, which contains two water molecules coordinated to a rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
center. In solid hydrates
In chemistry, a hydrate is a substance that contains water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the ...
, water can be either a ligand or simply lodged in the framework, or both. Thus, consists of e2(H2O)6sup>2+ centers and one "lattice water". Water is typically a monodentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals ...
ligand, i.e., it forms only one bond with the central atom.
Organic chemistry
As a hard base, water reacts readily with organic carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
s; for example in a hydration reaction
In chemistry, a hydration reaction is a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, che ...
, a hydroxyl group () and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When the addition of water to an organic molecule cleaves the molecule in two, hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
is said to occur. Notable examples of hydrolysis are the saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. ...
of fats and the digestion
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intest ...
of proteins and polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
. Water can also be a leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
in SN2 substitution and E2 elimination reactions; the latter is then known as a dehydration reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
Dehydration reactions in organic che ...
.
Water in redox reactions
Water contains hydrogen in the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
+1 and oxygen in the oxidation state −2. It oxidizes chemicals such as hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
, alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
metals, and some alkaline earth
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all s ...
metals. One example of an alkali metal reacting with water is:
:2 Na + 2 → + 2 + 2
Some other reactive metals, such as aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has ...
and beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form mi ...
, are oxidized by water as well, but their oxides adhere to the metal and form a passive
Passive may refer to:
* Passive voice, a grammatical voice common in many languages, see also Pseudopassive
* Passive language, a language from which an interpreter works
* Passivity (behavior), the condition of submitting to the influence of on ...
protective layer. Note that the rusting
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH ...
of iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
is a reaction between iron and oxygen that is dissolved in water, not between iron and water.
Water can be oxidized to emit oxygen gas, but very few oxidants react with water even if their reduction potential is greater than the potential of . Almost all such reactions require a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. An example of the oxidation of water is:
:4 + 2 → 4 AgF + 4 HF +
Electrolysis
Water can be split into its constituent elements, hydrogen, and oxygen, by passing an electric current through it. This process is called electrolysis. The cathode half reaction is:
:2 + 2 →
The anode half reaction is:
: 2 → + 4 + 4
The gases produced bubble to the surface, where they can be collected or ignited with a flame above the water if this was the intention. The required potential for the electrolysis of pure water is 1.23 V at 25 °C. The operating potential is actually 1.48 V or higher in practical electrolysis.
History
Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
showed that water was composed of oxygen and hydrogen in 1781. The first decomposition of water into hydrogen and oxygen, by electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
, was done in 1800 by English chemist William Nicholson and Anthony Carlisle
Sir Anthony Carlisle FRCS, FRS (15 February 1768 in Stillington, County Durham, England – 2 November 1840 in London) was an English surgeon.
Life
He was born in Stillington, County Durham, the third son of Thomas Carlisle and his first wife, ...
. In 1805, Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (, , ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen (with Alexander von Humboldt), for two laws ...
and Alexander von Humboldt
Friedrich Wilhelm Heinrich Alexander von Humboldt (14 September 17696 May 1859) was a German polymath, geographer, naturalist, explorer, and proponent of Romantic philosophy and science. He was the younger brother of the Prussian minister, p ...
showed that water is composed of two parts hydrogen and one part oxygen.
Gilbert Newton Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond a ...
isolated the first sample of pure heavy water in 1933.
The properties of water have historically been used to define various temperature scales. Notably, the Kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phys ...
, Celsius
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The ...
, Rankine Rankine is a surname. Notable people with the surname include:
* William Rankine (1820–1872), Scottish engineer and physicist
** Rankine body an elliptical shape of significance in fluid dynamics, named for Rankine
** Rankine scale, an absolute-te ...
, and Fahrenheit
The Fahrenheit scale () is a temperature scale based on one proposed in 1724 by the physicist Daniel Gabriel Fahrenheit (1686–1736). It uses the degree Fahrenheit (symbol: °F) as the unit. Several accounts of how he originally defined his ...
scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of Delisle, Newton
Newton most commonly refers to:
* Isaac Newton (1642–1726/1727), English scientist
* Newton (unit), SI unit of force named after Isaac Newton
Newton may also refer to:
Arts and entertainment
* ''Newton'' (film), a 2017 Indian film
* Newton ( ...
, Réaumur, and Rømer were defined similarly. The triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the subli ...
of water is a more commonly used standard point today.
Nomenclature
The accepted IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
name of water is ''oxidane'' or simply ''water'', or its equivalent in different languages, although there are other systematic names which can be used to describe the molecule. Oxidane is only intended to be used as the name of the mononuclear parent hydride
In chemistry, a parent hydride in IUPAC nomenclature refers to a main group compound with the formula , where A is a main group element. The names of parent hydrides end with ''-ane'', analogous with the nomenclature for alkanes. Derivatives of p ...
used for naming derivatives of water by substituent nomenclature. These derivatives commonly have other recommended names. For example, the name hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
is recommended over ''oxidanyl'' for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as tetrahydropyran
Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by addin ...
.
The simplest systematic name of water is ''hydrogen oxide''. This is analogous to related compounds such as hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
, hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
, and deuterium oxide (heavy water). Using chemical nomenclature for type I ionic binary compounds, water would take the name ''hydrogen monoxide'', but this is not among the names published by the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC). Another name is ''dihydrogen monoxide'', which is a rarely used name of water, and mostly used in the dihydrogen monoxide parody
The dihydrogen monoxide parody involves calling water by an unfamiliar chemical name, most often "dihydrogen monoxide" (DHMO), and listing some of water's properties in a particularly alarming manner, such as accelerating corrosion (rust) and ...
.
Other systematic names for water include ''hydroxic acid'', ''hydroxylic acid'', and ''hydrogen hydroxide'', using acid and base names. None of these exotic names are used widely. The polarized form of the water molecule, , is also called hydron Hydron has the following meanings:
* Hydron (chemistry)
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol . The general term "hydron", endorsed by the IUPAC, encompasses cati ...
hydroxide by IUPAC nomenclature.water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
, steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
, some form of ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaq ...
, or a component in a mixture or mineral.
See also
* Chemical bonding of water
Water () is a simple triatomic bent molecule with C2v molecular symmetry and bond angle of 104.5° between the central oxygen atom and the hydrogen atoms. Despite being one of the simplest triatomic molecules, its chemical bonding scheme is nonethe ...
* Dihydrogen monoxide parody
The dihydrogen monoxide parody involves calling water by an unfamiliar chemical name, most often "dihydrogen monoxide" (DHMO), and listing some of water's properties in a particularly alarming manner, such as accelerating corrosion (rust) and ...
* Double distilled water
Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water was, formerly, the most common form of purified water, but, in recent years, water is more frequently puri ...
* Electromagnetic absorption by water
The absorption of electromagnetic radiation by water depends on the state of the water.
The absorption in the gas phase occurs in three regions of the spectrum. Rotational transitions are responsible for absorption in the microwave and far-inf ...
* Fluid dynamics
In physics and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids— liquids and gases. It has several subdisciplines, including ''aerodynamics'' (the study of air and other gases in motion) an ...
* Hard water
Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbo ...
* Heavy water
* Hydrogen polyoxide
Hydrogen polyoxides (also known as oxidanes, oxohydrogens, or oxyhydrogens) are chemical compounds that consist only of hydrogen and oxygen atoms, are bonded exclusively by single bonds (i.e., they are saturated), and are acyclic (have molecular ...
* Ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaq ...
* Optical properties of water and ice The refractive index of water at 20 °C for visible light is 1.33. The refractive index of normal ice is 1.31 (from List of refractive indices). In general, an index of refraction is a complex number with real and imaginary parts, where the lat ...
* Steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
* Superheated water
Superheated water is liquid water under pressure at temperatures between the usual boiling point, and the critical temperature, . It is also known as "subcritical water" or "pressurized hot water". Superheated water is stable because of overpres ...
*
* Water cluster
In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Many such clusters have been predicted by theoretical models ( in silico), and some have been detected experimentally in various contexts suc ...
* Water (data page)
This page provides supplementary data to the article properties of water.
Further comprehensive authoritative data can be found at thNIST Webbookpage on thermophysical properties of fluids.
Structure and properties
Thermodynamic properties
...
* Water dimer
The water dimer consists of two water (molecule), water molecules loosely bound by a hydrogen bond. It is the smallest water cluster. Because it is the simplest model system for studying hydrogen bonding in water, it has been the target of many ...
* Water model
In computational chemistry, a water model is used to simulate and thermodynamically calculate water clusters, liquid water, and aqueous solutions with explicit solvent. The models are determined from quantum mechanics, molecular mechanics, experim ...
* Water thread experiment
The water thread experiment is a phenomenon that occurs when two containers of deionized water, placed on an insulator, are connected by a thread, then a high-voltage positive electric charge is applied to one container, and a negative charge to ...
Footnotes
References
Notes
Bibliography
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Further reading
*
External links
*
Release on the IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use
(simpler formulation)
*
* Calculation of ttp://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe?component=Water vapor pressurebr>liquid densitydynamic liquid viscosity
an
surface tension
of water
Water Density Calculator
NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research.
NASA was established in 1958, succeeding t ...
{{DEFAULTSORT:Properties Of Water
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
Forms of water
Hydrogen compounds
Oxygen compounds
Hydroxides
Inorganic solvents
Neutron moderators
Oxides
Limnology
Oceanography
Extraterrestrial water
Transport phenomena
Heat transfer
Greenhouse gases
 Water has a very high
Water has a very high  The density of saltwater depends on the dissolved salt content as well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 1.9 °C (see
The density of saltwater depends on the dissolved salt content as well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 1.9 °C (see  Water is
Water is 
 Water molecules stay close to each other ( cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high
Water molecules stay close to each other ( cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high 
 Water is an excellent
Water is an excellent