volume fraction on:
[Wikipedia]
[Google]
[Amazon]
In chemistry and
''Volume-volume percentage''Chembuddy
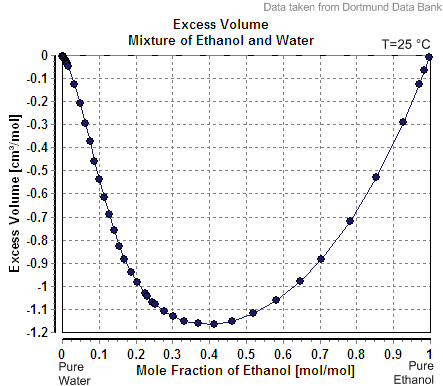 In the case of a mixture of ethanol and water, which are miscible in all proportions, the designation of solvent and solute is arbitrary. The volume of such a mixture is slightly less than the sum of the volumes of the components. Thus, by the above definition, the term "40% alcohol by volume" refers to a mixture of 40 volume units of ethanol with enough water to make a final volume of 100 units, rather than a mixture of 40 units of ethanol with 60 units of water. The "enough water" is actually slightly more than 60 volume units, since water-ethanol mixture loses volume due to intermolecular attraction.
In the case of a mixture of ethanol and water, which are miscible in all proportions, the designation of solvent and solute is arbitrary. The volume of such a mixture is slightly less than the sum of the volumes of the components. Thus, by the above definition, the term "40% alcohol by volume" refers to a mixture of 40 volume units of ethanol with enough water to make a final volume of 100 units, rather than a mixture of 40 units of ethanol with 60 units of water. The "enough water" is actually slightly more than 60 volume units, since water-ethanol mixture loses volume due to intermolecular attraction.
fluid mechanics
Fluid mechanics is the branch of physics concerned with the mechanics of fluids (liquids, gases, and plasmas) and the forces on them.
It has applications in a wide range of disciplines, including mechanical, aerospace, civil, chemical and ...
, the volume fraction φ''i'' is defined as the volume of a constituent ''V''''i'' divided by the volume of all constituents of the mixture ''V'' prior to mixing:
:
Being dimensionless
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1) ...
, its unit is 1; it is expressed as a number, e.g., 0.18. It is the same concept as volume percent (vol%) except that the latter is expressed with a denominator of 100, e.g., 18%.
The volume fraction coincides with the volume concentration in ideal solution
In chemistry, an ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing by definition; the closer to zero ...
s where the volumes of the constituents are additive (the volume of the solution is equal to the sum of the volumes of its ingredients).
The sum of all volume fractions of a mixture is equal to 1:
:
The volume fraction (percentage by volume, vol%) is one way of expressing the composition of a mixture with a dimensionless quantity
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1) ...
; mass fraction (percentage by weight, wt%) and mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ...
(percentage by moles, mol%) are others.
Volume concentration and volume percent
Volume percent is theconcentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
of a certain solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solve ...
, measured by volume, in a solution. It has as a denominator the volume of the mixture itself, as usual for expressions of concentration, rather than the total of all the individual component's volumes prior to mixing:
:
:
Volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). Th ...
percent is usually used when the solution is made by mixing two fluid
In physics, a fluid is a liquid, gas, or other material that continuously deforms (''flows'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are substances which cannot resist any shea ...
s, such as liquids or gases
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), ...
. However, percentages are only additive for ideal gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is ...
es.website
A website (also written as a web site) is a collection of web pages and related content that is identified by a common domain name and published on at least one web server. Examples of notable websites are Google, Facebook, Amazon, and Wikip ...
The percentage by volume (vol%) is one way of expressing the composition of a mixture with a dimensionless quantity
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1) ...
; mass fraction (percentage by weight, wt%) and mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ...
(percentage by moles, mol%) are others.
 In the case of a mixture of ethanol and water, which are miscible in all proportions, the designation of solvent and solute is arbitrary. The volume of such a mixture is slightly less than the sum of the volumes of the components. Thus, by the above definition, the term "40% alcohol by volume" refers to a mixture of 40 volume units of ethanol with enough water to make a final volume of 100 units, rather than a mixture of 40 units of ethanol with 60 units of water. The "enough water" is actually slightly more than 60 volume units, since water-ethanol mixture loses volume due to intermolecular attraction.
In the case of a mixture of ethanol and water, which are miscible in all proportions, the designation of solvent and solute is arbitrary. The volume of such a mixture is slightly less than the sum of the volumes of the components. Thus, by the above definition, the term "40% alcohol by volume" refers to a mixture of 40 volume units of ethanol with enough water to make a final volume of 100 units, rather than a mixture of 40 units of ethanol with 60 units of water. The "enough water" is actually slightly more than 60 volume units, since water-ethanol mixture loses volume due to intermolecular attraction.
Relation to mass fraction
Volume fraction is related to mass fraction, by where is the constituent density, and is the mixture density.See also
*Alcohol by volume
Alcohol by volume (abbreviated as ABV, abv, or alc/vol) is a standard measure of how much alcohol (ethanol) is contained in a given volume of an alcoholic beverage (expressed as a volume percent). It is defined as the number of millilitres (mL) o ...
* Alcohol meter
* Alcohol proof
* Apparent molar property
* For non-ideal mixtures, see Partial molar volume and Excess molar quantity
* Percentage
In mathematics, a percentage (from la, per centum, "by a hundred") is a number or ratio expressed as a fraction of 100. It is often denoted using the percent sign, "%", although the abbreviations "pct.", "pct" and sometimes "pc" are also ...
* Mass fraction (chemistry)
References
{{Chemical solutions Dimensionless numbers of chemistry Physical chemistry Thermodynamics Analytical chemistry