|
Apparent Molar Property
In thermodynamics, an apparent molar property of a solution component in a mixture or solution is a quantity defined with the purpose of isolating the contribution of each component to the non-ideality of the mixture. It shows the change in the corresponding solution property (for example, volume) per mole of that component added, when all of that component is added to the solution. It is described as ''apparent'' because it appears to represent the molar property of that component ''in solution'', provided that the properties of the other solution components are assumed to remain constant during the addition. However this assumption is often not justified, since the values of apparent molar properties of a component may be quite different from its molar properties in the pure state. For instance, the volume of a solution containing two components identified as solvent and solute is given by : V=V_0 + ^\phi_1 \ =\tilde_ n_ + ^\phi\tilde_1 n_1 \, where is the volume of the pure s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NaCl
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
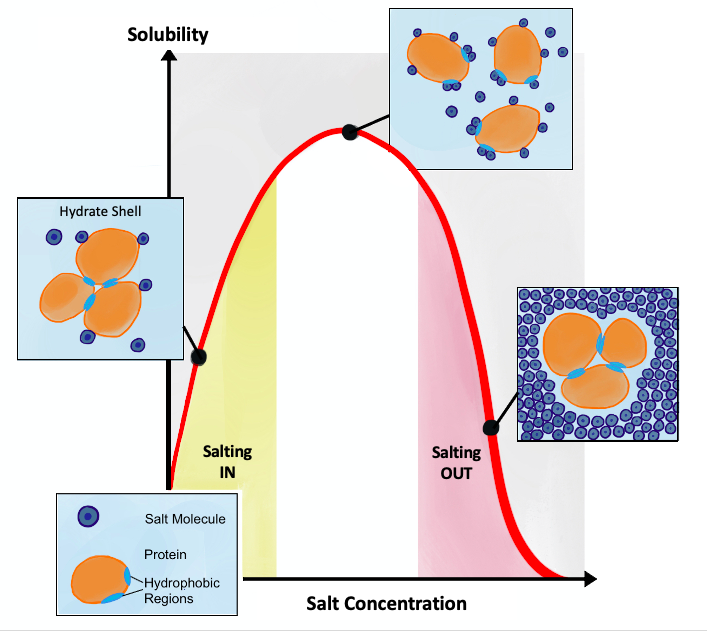
Salting Out
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salting In
Salting in refers to the effect where increasing the ionic strength of a solution increases the solubility of a solute, such as a protein. This effect tends to be observed at lower ionic strengths. Protein solubility is a complex function of physicochemical nature of the protein, pH, temperature, and the concentration of the salt used. It also depends on whether the salt is kosmotropic, whereby the salt will stabilize water. The solubility of proteins usually increases slightly in the presence of salt, referred to as "salting in". However, at high concentrations of salt, the solubility of the proteins drop sharply and proteins can precipitate out, referred to as "salting out". Anionic interactions Initial salting in at low concentrations is explained by the Debye–Huckel theory. Proteins are surrounded by the salt counterions (ions of opposite net charge) and this screening results in decreasing electrostatic free energy of the protein and increasing activity of the solvent, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Solution
In chemistry, an ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing by definition; the closer to zero the enthalpy of mixing is, the more "ideal" the behavior of the solution becomes. The vapor pressures of the solvent and solute obey Raoult's law and Henry's law, respectively, and the activity coefficient (which measures deviation from ideality) is equal to one for each component. The concept of an ideal solution is fundamental to chemical thermodynamics and its applications, such as the explanation of colligative properties. Physical origin Ideality of solutions is analogous to ideality for gases, with the important difference that intermolecular interactions in liquids are strong and cannot simply be neglected as they can for ideal gases. Instead we assume that the mean strength of the interactions are the same between all the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol (data Page)
This page provides supplementary chemical data on ethanol. Material Safety Data Sheet External MSDS Structure and properties Thermodynamic properties Spectral data Vapor pressure of liquid Density of ethanol at various temperatures Data obtained from These data correlate as ρ /cm3= −8.461834 ''T'' �C+ 0.8063372 with an ''R''2 = 0.99999. Properties of aqueous ethanol solutions Data obtained from Boiling points of aqueous solutions Data obtained from ''CRC Handbook of Chemistry (Page 2117)'' ‡ Azeotropic mixture Charts References * * {{DEFAULTSORT:Ethanol (data page) Chemical data pages Data page Chemical data pages cleanup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Percent
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt%''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the total mass of a sample ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
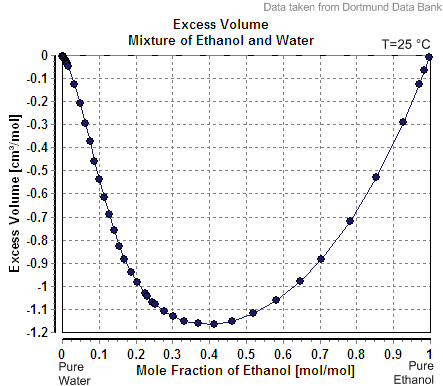
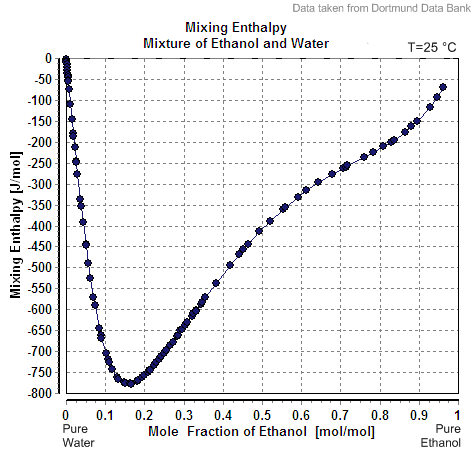
Excess Volume Mixture Of Ethanol And Water
Excess may refer to: * Angle excess, in spherical trigonometry * Insurance excess, similar to a deductible * Excess, in chemistry, a reagent that is not the limiting reagent * "Excess", a song by Tricky from the album '' Blowback'' * ''Excess'' (album), an album by Coma See also * Excess-K Offset binary, also referred to as excess-K, excess-''N'', excess-e, excess code or biased representation, is a method for signed number representation where a signed number n is represented by the bit pattern corresponding to the unsigned numbe ..., or offset binary, in computing * XS (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benton Owen
Benton may refer to: Places Canada *Benton, a local service district south of Woodstock, New Brunswick *Benton, Newfoundland and Labrador United Kingdom * Benton, Devon, near Bratton Fleming * Benton, Tyne and Wear United States *Benton, Alabama *Benton, Arkansas *Benton, California *Benton, Illinois *Benton, Indiana * Benton, Iowa *Benton, Kansas *Benton, Kentucky *Benton, Louisiana * Benton, Maine * Benton, Michigan *Benton, Missouri * Benton, New Hampshire * Benton, New York * Benton, Ohio * Benton, Pennsylvania (other) *Benton, Tennessee *Benton, Wisconsin * Benton (town), Wisconsin * Benton (Middleburg, Virginia), a historic house * Benton Charter Township, Michigan * Benton Crossing, California *Benton Harbor, Michigan * Benton Hot Springs, California (ghost town) * Benton Ridge, Ohio *Fort Benton, Montana *Lake Benton, Minnesota *Utu Utu Gwaitu Paiute Tribe of the Benton Paiute Reservation, California People * Benton (surname) Other * The Benton meteorite of 194 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbert Harned
Herbert may refer to: People Individuals * Herbert (musician), a pseudonym of Matthew Herbert Name * Herbert (given name) * Herbert (surname) Places Antarctica * Herbert Mountains, Coats Land * Herbert Sound, Graham Land Australia * Herbert, Northern Territory, a rural locality * Herbert, South Australia. former government town * Division of Herbert, an electoral district in Queensland * Herbert River, a river in Queensland * County of Herbert, a cadastral unit in South Australia Canada * Herbert, Saskatchewan, Canada, a town * Herbert Road, St. Albert, Canada New Zealand * Herbert, New Zealand, a town * Mount Herbert (New Zealand) United States * Herbert, Illinois, an unincorporated community * Herbert, Michigan, a former settlement * Herbert Creek, a stream in South Dakota * Herbert Island, Alaska Arts, entertainment, and media Fictional entities * Herbert (Disney character) * Herbert Pocket (''Great Expectations'' character), Pip's close friend and roommate in the Cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |