Vitamin B12 total synthesis on:
[Wikipedia]
[Google]
[Amazon]
The
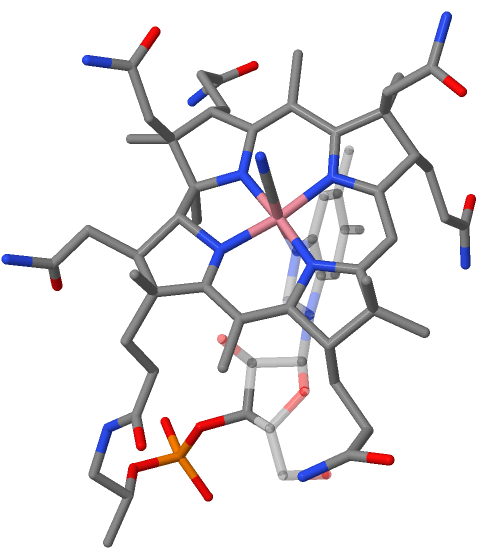 Vitamin B12, C63H88CoN14O14P, is the most complex of all known
Vitamin B12, C63H88CoN14O14P, is the most complex of all known  Several natural variants of the B12 structure exist that differ in these axial ligands. In the vitamin itself, the cobalt bears a
Several natural variants of the B12 structure exist that differ in these axial ligands. In the vitamin itself, the cobalt bears a

 Already in 1960, the research group of the biochemist in
Already in 1960, the research group of the biochemist in
 Early in 1967, the Harvard group accomplished the synthesis of the model A-D-component, with the f-side chain undifferentiated, bearing a methyl ester function like all other side chains. From then on, the two groups systematically exchanged samples of their respective halves of the corrinoid target structure. By 1970, they had collaboratively connected Harvard's undifferentiated A-D-component with ETH's B-C-component, producing dicyano-cobalt(III)-5,15-bisnor-heptamethyl-cobyrinate 1 (fig. 4). The ETH group identified this totally synthetic corrinoid intermediate by direct comparison with a sample produced from natural vitamin B12.
In this advanced model study, reaction conditions for the demanding processes of the C/D-coupling and the A/B-cyclization via sulfide contraction method were established. Those for the C/D-coupling were successfully explored in both laboratories, the superior conditions were those found at Harvard, while the method for the A/B-ring closure via an intramolecular version of the sulfide contraction was developed at ETH. Later it was shown at Harvard that the A/B-ring closure could also be achieved by thio-iminoester/enamine condensation.
By early 1971, the Harvard group had accomplished the synthesis of the final A-D-component, containing the f-side chain carboxyl function at ring D differentiated from all the carboxyl functions as a nitrile group (as shown in 2 in fig. 4; see also fig. 3). The A/D-part of the B12 structure incorporates the constitutionally and configurationally most intricate part of the vitamin molecule; its synthesis is regarded as the
Early in 1967, the Harvard group accomplished the synthesis of the model A-D-component, with the f-side chain undifferentiated, bearing a methyl ester function like all other side chains. From then on, the two groups systematically exchanged samples of their respective halves of the corrinoid target structure. By 1970, they had collaboratively connected Harvard's undifferentiated A-D-component with ETH's B-C-component, producing dicyano-cobalt(III)-5,15-bisnor-heptamethyl-cobyrinate 1 (fig. 4). The ETH group identified this totally synthetic corrinoid intermediate by direct comparison with a sample produced from natural vitamin B12.
In this advanced model study, reaction conditions for the demanding processes of the C/D-coupling and the A/B-cyclization via sulfide contraction method were established. Those for the C/D-coupling were successfully explored in both laboratories, the superior conditions were those found at Harvard, while the method for the A/B-ring closure via an intramolecular version of the sulfide contraction was developed at ETH. Later it was shown at Harvard that the A/B-ring closure could also be achieved by thio-iminoester/enamine condensation.
By early 1971, the Harvard group had accomplished the synthesis of the final A-D-component, containing the f-side chain carboxyl function at ring D differentiated from all the carboxyl functions as a nitrile group (as shown in 2 in fig. 4; see also fig. 3). The A/D-part of the B12 structure incorporates the constitutionally and configurationally most intricate part of the vitamin molecule; its synthesis is regarded as the
 Starting in fall of 1969 with the B-C-component of the A/B approach and a ring-D precursor prepared from the
Starting in fall of 1969 with the B-C-component of the A/B approach and a ring-D precursor prepared from the
 The first decisive identification of a totally synthetic intermediate on the way to cobyric acid was carried out in February 1972 with a crystalline sample of totally synthetic dicyano-cobalt(III)-hexamethyl-cobyrinate-f-amide 3 (fig. 6), found to be identical in all data with a crystalline relay sample made from vitamin B12 by methanolysis to cobester 4, followed by partial ammonolysis and separation of the resulting mixture. At the time when Woodward announced the "Total Synthesis of Vitamin B12" at the IUPAC conference in New Delhi in February 1972, the totally synthetic sample of the f-amide was one that had been made at ETH by the photochemical A/D approach, while the first sample of synthetic cobyric acid, identified with natural cobyric acid, had been obtained at Harvard by partial synthesis from B12-derived f-amide relay material. Thus, the Woodward/Eschenmoser achievement around that time had been, strictly speaking, two formal total syntheses of cobyric acid, as well as two formal total syntheses of the vitamin.
In the later course of 1972, two crystalline
The first decisive identification of a totally synthetic intermediate on the way to cobyric acid was carried out in February 1972 with a crystalline sample of totally synthetic dicyano-cobalt(III)-hexamethyl-cobyrinate-f-amide 3 (fig. 6), found to be identical in all data with a crystalline relay sample made from vitamin B12 by methanolysis to cobester 4, followed by partial ammonolysis and separation of the resulting mixture. At the time when Woodward announced the "Total Synthesis of Vitamin B12" at the IUPAC conference in New Delhi in February 1972, the totally synthetic sample of the f-amide was one that had been made at ETH by the photochemical A/D approach, while the first sample of synthetic cobyric acid, identified with natural cobyric acid, had been obtained at Harvard by partial synthesis from B12-derived f-amide relay material. Thus, the Woodward/Eschenmoser achievement around that time had been, strictly speaking, two formal total syntheses of cobyric acid, as well as two formal total syntheses of the vitamin.
In the later course of 1972, two crystalline

 As a joint full publication of the syntheses by the Harvard and ETH groups (announced in and expected in) had not appeared by 1977, an article describing the final version of the photochemical A/D approach already accomplished in 1972 was published 1977 in Science. This article is an extended English translation of one that had already appeared 1974 in Naturwissenschaften, based on a lecture given by Eschenmoser on January 21, 1974 at a meeting of the Zürcher Naturforschende Gesellschaft. Four decades later, in 2015, the same author finally published a series of six full papers describing the work of the ETH group on
As a joint full publication of the syntheses by the Harvard and ETH groups (announced in and expected in) had not appeared by 1977, an article describing the final version of the photochemical A/D approach already accomplished in 1972 was published 1977 in Science. This article is an extended English translation of one that had already appeared 1974 in Naturwissenschaften, based on a lecture given by Eschenmoser on January 21, 1974 at a meeting of the Zürcher Naturforschende Gesellschaft. Four decades later, in 2015, the same author finally published a series of six full papers describing the work of the ETH group on
99
136, title=Classics in Total Synthesis: Targets, Strategies, Methods, url=https://archive.org/details/classicstotalmet00kcni_087, url-access=limited, publisher=VCH Verlag Chemie, location=Weinheim {{cite journal, doi=10.1021/ed075p1225, title=The Art and Science of Organic and Natural Products Synthesis, journal=Journal of Chemical Education, volume=75, issue=10, pages=1225–1258, date=1998, last1=Nicolaou, first1=K. C., author-link1=K. C. Nicolaou, last2=Sorensen, first2=E. J., last3=Winssinger, first3=N., bibcode=1998JChEd..75.1225N, doi-access=free {{cite journal, doi=10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L, pmid=10649349, title=The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century, journal=Angewandte Chemie International Edition, volume=39, issue=1, pages=44–122, date=2000, last1=Nicolaou, first1=K. C., author-link1=K. C. Nicolaou, last2=Vourloumis, first2=Dionisios, last3=Winssinger, first3=Nicolas, last4=Baran, first4=Phil S., author-link4=Phil S. Baran {{cite journal, doi=10.1002/1099-0690(200301)2003:1<30::AID-EJOC30>3.0.CO;2-I, title=Total Synthesis of Cobyric Acid: Historical Development and Recent Synthetic Innovations, journal=European Journal of Organic Chemistry, volume=2003, pages=30–45, date=2003, last1=Riether, first1=Doris, last2=Mulzer, first2=Johann, author-link2=Johann Mulzer {{cite journal, doi=10.1007/s12045-014-0064-4, title=Eschenmoser approach to vitamin B12 by A/D strategy, journal=Resonance, volume=19, issue=7, pages=624–640, date=2014, last1=Craig, first1=G. Wayne, s2cid=118161709 {{cite journal, doi=10.1142/S1088424615500960, title=Total synthesis of vitamin B12 - a fellowship of the ring, journal=Journal of Porphyrins and Phthalocyanines, volume=20, pages=1–20, date=2016, last1=Craig, first1=G. Wayne {{cite journal, doi=10.1126/science.1067545, pmid=11729290, title=Natural product synthesis: the art of total synthesis, journal=Science, volume=294, issue=5548, pages=1842–1843, date=2001, last1=Marko, first1=I. E., s2cid=22467000 {{cite journal, doi=10.1039/qr9712500031, title=Recent developments in the chemistry of pyrrolic compounds, journal=Quarterly Reviews, Chemical Society, volume=25, pages=31–85, date=1971, last1=Smith, first1=K. M. {{cite book, doi=10.1142/9789814397605_0020, last1=Montforts, first1=Franz-Peter, last2=Osmers, first2=Martina, last3=Leupold, first3=Dennis, chapter=Chemical Synthesis of Artificial Corrins, editor1-last=Kadish, editor1-first=Karl M., editor2-last=Smith, editor2-first=Kevin M., editor3-last=Guilard, editor3-first=Roger, title=Handbook of Porphyrin Science, volume=25, date=2012, pages=265–307, publisher=World Scientific Publishing, isbn=978-981-4397-66-7 {{cite web, url=https://www.research-collection.ethz.ch/, title=ETH Research Collection (previously ETH e-collection), publisher=
total synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ...
of the complex biomolecule vitamin B12 was accomplished in two different approaches by the collaborating research groups of Robert Burns Woodward
Robert Burns Woodward (April 10, 1917 – July 8, 1979) was an American organic chemist. He is considered by many to be the most preeminent synthetic organic chemist of the twentieth century, having made many key contributions to the subject, e ...
at Harvard
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of higher le ...
and Albert Eschenmoser
Albert Jakob Eschenmoser (born 5 August 1925) is a Swiss organic chemist, best known for his work on the synthesis of complex heterocyclic natural compounds, most notably vitamin B12. In addition to his significant contributions to the field of ...
at ETH
(colloquially)
, former_name = eidgenössische polytechnische Schule
, image = ETHZ.JPG
, image_size =
, established =
, type = Public
, budget = CHF 1.896 billion (2021)
, rector = Günther Dissertori
, president = Joël Mesot
, a ...
in 1972. The accomplishment required the effort of no less than 91 postdoctoral researcher
A postdoctoral fellow, postdoctoral researcher, or simply postdoc, is a person professionally conducting research after the completion of their doctoral studies (typically a PhD). The ultimate goal of a postdoctoral research position is to p ...
s (Harvard: 77, ETH: 14), and 12 Ph.D. students (at ETH) from 19 different nations over a period of almost 12 years. The synthesis project induced and involved a major change of paradigm
In science and philosophy, a paradigm () is a distinct set of concepts or thought patterns, including theories, research methods, postulates, and standards for what constitute legitimate contributions to a field.
Etymology
''Paradigm'' comes f ...
in the field of natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
.
The molecule
 Vitamin B12, C63H88CoN14O14P, is the most complex of all known
Vitamin B12, C63H88CoN14O14P, is the most complex of all known vitamins
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an essential micronutrient that an organism needs in small quantities for the proper functioning of its metabolism. Essential nutrien ...
. Its chemical structure had been determined by x-ray crystal structure analysis in 1956 by the research group of Dorothy Hodgkin
Dorothy Mary Crowfoot Hodgkin (née Crowfoot; 12 May 1910 – 29 July 1994) was a Nobel Prize-winning British chemist who advanced the technique of X-ray crystallography to determine the structure of biomolecules, which became essential fo ...
(Oxford University
Oxford () is a city in England. It is the county town and only city of Oxfordshire. In 2020, its population was estimated at 151,584. It is north-west of London, south-east of Birmingham and north-east of Bristol. The city is home to the ...
) in collaboration with Kenneth N. Trueblood at UCLA
The University of California, Los Angeles (UCLA) is a public land-grant research university in Los Angeles, California. UCLA's academic roots were established in 1881 as a teachers college then known as the southern branch of the California St ...
and John G. White at Princeton University
Princeton University is a private university, private research university in Princeton, New Jersey. Founded in 1746 in Elizabeth, New Jersey, Elizabeth as the College of New Jersey, Princeton is the List of Colonial Colleges, fourth-oldest ins ...
.
Core of the molecule is the corrin
Corrin is a heterocyclic compound. It is the parent macrocycle related to the substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins).Nelson, D. L.; Cox, M. M. "Lehninger, Princ ...
structure, a nitrogenous tetradentate ligand
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands.
Tetradentate ligands are ...
system. This is biogenetically related to porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
s and chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
s, yet differs from them in important respects: the carbon skeleton lacks one of the four meso carbons between the five-membered rings, two rings (A and D, fig. 1) being directly connected by a carbon-carbon single bond. The corrin chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
system is thus non-cyclic and expands over three meso positions only, incorporating three vinylogous
In organic chemistry, vinylogy is the transmission of electronic effects through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoaldehyde ...
amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
units. Lined up at the periphery of the macrocyclic
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
ring are eight methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
groups and four propionic and three acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
side chains. Nine carbon atoms on the corrin periphery are chirogenic centers. The tetradentate, monobasic corrin ligand is equatorially coordinated with a trivalent cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
ion which bears two additional axial ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s.
cyano
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
group on the top side of the corrin plane (cyanocobalamin
Cyanocobalamin is a form of vitamin used to treat vitamin deficiency except in the presence of cyanide toxicity. The deficiency may occur in pernicious anemia, following surgical removal of the stomach, with fish tapeworm, or due to bowel ...
), and a nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules wi ...
loop on the other. This loop is connected on its other end to the peripheral propionic amide group at ring D and consists of structural elements derived from aminopropanol, phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
, ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compo ...
, and 5,6-dimethylbenzimidazole. One of the nitrogen atoms of the imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole Diazole refers ...
ring is axially coordinated to the cobalt, the nucleotide loop thus forming a nineteen-membered ring. All side chain carboxyl groups are amides.
Cobyric acid, one of the natural derivatives of vitamin B12, lacks the nucleotide loop; depending on the nature of the two axial ligands, it displays instead its propionic acid function at ring D as carboxylate (as shown in fig. 1), or carboxylic acid (with two cyanide ligands at cobalt).
The two syntheses
The structure of vitamin B12 was the first low-molecular weightnatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
determined by x-ray analysis rather than by chemical degradation. Thus, while the structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
of this novel type of complex biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
was established, its chemistry remained essentially unknown; exploration of this chemistry became one of the tasks of the vitamin's chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In moder ...
. In the 1960s, synthesis of such an exceptionally complex and unique structure presented the major challenge at the frontier of research in organic natural product synthesis.
Stuttgart
Stuttgart (; Swabian: ; ) is the capital and largest city of the German state of Baden-Württemberg. It is located on the Neckar river in a fertile valley known as the ''Stuttgarter Kessel'' (Stuttgart Cauldron) and lies an hour from the ...
had reconstituted vitamin B12 from one of its naturally occurring derivatives, cobyric acid, by stepwise construction of the vitamin's nucleotide loop. This work amounted to a partial synthesis of vitamin B12 from a natural product containing all the structural elements of vitamin B12 except the nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules wi ...
loop. Therefore, cobyric acid was chosen as the target molecule for a total synthesis of vitamin B12.
Collaborative work of research groups at Harvard
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of higher le ...
and at ETH
(colloquially)
, former_name = eidgenössische polytechnische Schule
, image = ETHZ.JPG
, image_size =
, established =
, type = Public
, budget = CHF 1.896 billion (2021)
, rector = Günther Dissertori
, president = Joël Mesot
, a ...
resulted in two cobyric acid syntheses, both concomitantly accomplished in 1972, one at Harvard, and the other at ETH. A "competitive collaboration" of that size, involving 103 graduate students and postdoctoral researchers for a total almost 177 person-years, is so far unique in the history of organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. The two syntheses are intricately intertwined chemically, yet they differ basically in the way the central macrocyclic
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
corrin ligand system is constructed. Both strategies are patterned after two model corrin syntheses developed at ETH. The first, published in 1964, achieved the construction of the corrin chromophore by combining an A-D-component with a B-C-component via iminoester/enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and th ...
-C,C-condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
s, the final corrin-ring closure being attained between rings A and B. The second model synthesis, published 1969, explored a novel photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
cycloisomerization process to create the direct A/D-ring junction as final corrin-ring closure between rings A and D.
The A/B approach to the cobyric acid syntheses was collaboratively pursued and accomplished in 1972 at Harvard. It combined a bicyclic Harvard A-D-component with an ETH B-C-component, and closed the macrocyclic corrin ring between rings A and B. The A/D approach to the synthesis, accomplished at ETH and finished at the same time as the A/B approach also in 1972, successively adds rings D and A to the B-C-component of the A/B approach and attains the corrin ring closure between rings A and D. The paths of the two syntheses met in a common corrinoid intermediate. The final steps from this intermediate to cobyric acid were carried out in the two laboratories again collaboratively, each group working with material prepared via their own approach, respectively.
Synopsis of the Harvard/ETH collaboration
The beginnings
Woodward and Eschenmoser embarked on the project of a chemical synthesis of vitamin B12 independently from each other. The ETH group started with a model study on how to synthesize a corrin ligand system in December 1959. In August 1961, the Harvard group began attacking the buildup of the B12 structure directly by aiming at the most complex part of the B12 molecule, the "western half" that contains the direct junction between rings A und D (the A-D-component). Already in October 1960, the ETH group had commenced the synthesis of a ring-B precursor of vitamin B12. At the beginning, progress at Harvard was rapid, until an unexpected stereochemical course of a central ring formation step interrupted the project. Woodward's recognition of the stereochemical enigma that came to light by the irritating behavior of one of his carefully planned synthetic steps became, according to his own writings, part of the developments that led to the orbital symmetry rules. After 1965, the Harvard group continued work towards an A-D-component along a modified plan, using (−)-camphor as the source of ring D.Joining forces: the A/B approach to cobyric acid synthesis
By 1964, the ETH group had accomplished the firstcorrin
Corrin is a heterocyclic compound. It is the parent macrocycle related to the substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins).Nelson, D. L.; Cox, M. M. "Lehninger, Princ ...
model synthesis, and also the preparation of a ring-B precursor as part of a construction of the B12 molecule itself. Since independent progress of the two groups towards their long-term objective was so clearly complementary, Woodward and Eschenmoser decided in 1965 to join forces and to pursue from then on the project of a B12 synthesis collaboratively, planning to utilize the ligand construction (ring coupling of components) strategy of the ETH model system.
By 1966, the ETH group had succeeded in synthesizing the B-C-component ("eastern half") by coupling their ring-B precursor to the ring-C precursor. The latter had also been prepared at Harvard from (−)-camphor by a strategy conceived and used earlier by A. Pelter and J. W. Cornforth in 1961. At ETH, the synthesis of the B-C-component involved the implementation of the C,C-condensation reaction via sulfide contraction. This newly developed method turned out to provide a general solution to the problem of constructing the characteristic structural elements of the corrin chromophore, the vinylogous amidine systems bridging the four peripheral rings.
apotheosis
Apotheosis (, ), also called divinization or deification (), is the glorification of a subject to divine levels and, commonly, the treatment of a human being, any other living thing, or an abstract idea in the likeness of a deity. The term has ...
of the Woodwardian art in natural product total synthesis.
The alternative approach to cobyric acid synthesis
As far back as 1966, the ETH group had started to explore, once again in a model system, an alternative strategy of corrin synthesis in which the corrin ring would be closed between rings A and D. The project was inspired by the conceivable existence of a thus far unknown bond reorganisation process. This if existing would make possible the construction of cobyric acid from one single starting material. Importantly, the hypothetical process, being interpreted as implying two sequential rearrangements, was recognized to be formally covered by the new reactivity classifications of sigmatropic rearrangements and electrocyclizations propounded by Woodward andHoffmann
Hoffmann is a German surname.
People A
* Albert Hoffmann (1846–1924), German horticulturist
* Alexander Hoffmann (born 1975), German politician
* Arthur Hoffmann (politician) (1857–1927), Swiss politician and member of the Swiss Federal Cou ...
in the context of their orbital symmetry rules!
By May 1968, the ETH group had demonstrated in a model study that the envisaged process, a photochemical A/D-seco-corrinate→corrinate cycloisomerization, does in fact exist. This process was first found to proceed with the Pd complex, but not at all with corresponding Ni(II)- or cobalt(III)-A/D-seco-corrinate complexes. It also went smoothly in complexes of metal ions such as zinc and other photochemically inert and loosely bound metal ions. These, after ring closure, could easily be replaced by cobalt. These discoveries opened the door to what eventually became the photochemical A/D approach of cobyric acid synthesis.
enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
of the starting material leading to the ring-B precursor, it took PhD student Walter Fuhrer less than one and a half years to translate the photochemical model corrin synthesis into a synthesis of dicyano-cobalt(III)-5,15-bisnor-a,b,d,e,g-pentamethyl-cobyrinate-c-''N,N''-dimethylamide-f-nitrile 2 ( fig. 4), the common corrinoid intermediate on the way to cobyric acid. At Harvard, the very same intermediate 2 was obtained around the same time by coupling the ring-D differentiated Harvard A-D-component (available in spring 1971) with the ETH B-C-component, applying the condensation methods developed earlier using the undifferentiated A-D-component.
Thus, in spring 1971, two different routes to a common corrinoid intermediate 2 ( fig. 4) along the way to cobyric acid had become available, one requiring 62 chemical steps ( Harvard/ETH A/B approach), the other 42 ( ETH A/D approach). In both approaches, the four peripheral rings derived from enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
precursors possessing the correct sense of chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
, thereby circumventing major stereochemical problems in the buildup of the ligand system. In the construction of the A/D-junction by the A/D-secocorrin→corrin
Corrin is a heterocyclic compound. It is the parent macrocycle related to the substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins).Nelson, D. L.; Cox, M. M. "Lehninger, Princ ...
cycloisomerization, formation of two A/D-diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
s had to be expected. Using cadmium(II) as the coordinating metal ion led to a very high diastereoselectivity in favor of the natural A/D-trans-isomer.
Once the corrin structure was formed by either approach, the three C-H- chirogenic centers at the periphery adjacent to the chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
system turned out to be prone to epimerizations with exceptional ease. This required a separation of diastereomers after most of the chemical steps in this advanced stage of the syntheses. It was fortunate indeed that, just around that time, the technique of high pressure liquid chromatography (HPLC) had been developed in analytical chemistry. HPLC became an indispensable tool in both laboratories; its use in the B12 project, pioneered by Jakob Schreiber at ETH, was the earliest application of the technique in natural product synthesis.
The joint final steps
The final conversion of the common corrinoid intermediate 2 (fig. 6) from the two approaches into the target cobyric acid required the introduction of the two missing methyl groups at the meso positions of the corrin chromophore between rings A/B and C/D, as well as theconversion
Conversion or convert may refer to:
Arts, entertainment, and media
* "Conversion" (''Doctor Who'' audio), an episode of the audio drama ''Cyberman''
* "Conversion" (''Stargate Atlantis''), an episode of the television series
* "The Conversion" ...
of all peripheral carboxyl functions into their amide form, except the critical carboxyl at the ring-D f-side chain (see fig. 6). These steps were collaboratively explored in strictly parallel fashion in both laboratories, the Harvard group using material produced via the A/B approach, the ETH group such prepared by the photochemical A/D approach.
epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is ...
s of totally synthetic dicyano-cobalt(III)-hexamethyl-cobyrinate-f-amide 3, as well as two crystalline epimers of the totally synthetic f-nitrile, all prepared via both synthetic approaches, were stringently identified chromatographically and spectroscopically with the corresponding B12-derived substances. At Harvard, cobyric acid was then made also from totally synthetic f-amide 3 prepared via the A/B approach. Finally, in 1976 at Harvard, totally synthetic cobyric acid was converted into vitamin B12 via the pathway pioneered by .
The publication record
Over the almost 12 years it took the two groups to reach their goal, both Woodward and Eschenmoser periodically reported on the stage of the collaborative project in lectures, some of them appearing in print. Woodward discussed the A/B approach in lectures published in 1968, and 1971, culminating in the announcement of the "Total Synthesis of Vitamin B12" in New Delhi in February 1972 published in 1973. This publication, and lectures with the same title Woodward delivered in the later part of the year 1972 are confined to the A/B approach of the synthesis and do not discuss the ETH A/D approach. Eschenmoser had discussed the ETH contributions to the A/B approach in 1968 at the 22ndRobert A. Welch Foundation The Welch Foundation, based in Houston, Texas, is one of the United States' oldest and largest private funding sources for chemistry researchers. It is a non-profit organization named for Robert Alonzo Welch, an industrialist who provided the funds ...
conference in Houston, as well as in his 1969 RSC Centenary Lecture "Roads to Corrins", published in 1970. He presented the ETH photochemical A/D approach to the B12 synthesis at the 23rd IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
Congress in Boston in 1971. The Zürich group announced the accomplishment of the synthesis of cobyric acid by the photochemical A/D-approach in two lectures delivered by PhD students Maag and Fuhrer at the Swiss Chemical Society Meeting in April 1972, Eschenmoser presented a lecture "Total Synthesis of Vitamin B12: the Photochemical Route" for the first time as Wilson Baker Lecture at the University of Bristol, Bristol/UK on May 8th, 1972.

 As a joint full publication of the syntheses by the Harvard and ETH groups (announced in and expected in) had not appeared by 1977, an article describing the final version of the photochemical A/D approach already accomplished in 1972 was published 1977 in Science. This article is an extended English translation of one that had already appeared 1974 in Naturwissenschaften, based on a lecture given by Eschenmoser on January 21, 1974 at a meeting of the Zürcher Naturforschende Gesellschaft. Four decades later, in 2015, the same author finally published a series of six full papers describing the work of the ETH group on
As a joint full publication of the syntheses by the Harvard and ETH groups (announced in and expected in) had not appeared by 1977, an article describing the final version of the photochemical A/D approach already accomplished in 1972 was published 1977 in Science. This article is an extended English translation of one that had already appeared 1974 in Naturwissenschaften, based on a lecture given by Eschenmoser on January 21, 1974 at a meeting of the Zürcher Naturforschende Gesellschaft. Four decades later, in 2015, the same author finally published a series of six full papers describing the work of the ETH group on corrin
Corrin is a heterocyclic compound. It is the parent macrocycle related to the substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins).Nelson, D. L.; Cox, M. M. "Lehninger, Princ ...
synthesis. Part I of the series contains a chapter entitled "The Final Phase of the Harvard/ETH Collaboration on the Synthesis of Vitamin B12", in which the contributions of the ETH group to the collaborative work on the synthesis of vitamin B12 between 1965 and 1972 are recorded.
The entire ETH
(colloquially)
, former_name = eidgenössische polytechnische Schule
, image = ETHZ.JPG
, image_size =
, established =
, type = Public
, budget = CHF 1.896 billion (2021)
, rector = Günther Dissertori
, president = Joël Mesot
, a ...
work is documented in full experimental detail in publicly accessible Ph.D. theses, almost 1'900 pages, all in German. Contributions of the 14 postdoctoral ETH researchers involved in the cobyric acid syntheses are mostly integrated in these theses. The detailed experimental work at Harvard
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of higher le ...
was documented in reports by the 77 postdoctoral researchers involved, with a total volume of more than 3'000 pages.
Representative reviews of the two approaches to the chemical synthesis of vitamin B12 have been published in detail by A. H. Jackson and K. M. Smith, T. Goto, R. V. Stevens, K. C. Nicolaou
Kyriacos Costa Nicolaou ( el, Κυριάκος Κ. Νικολάου; born July 5, 1946) is a Cypriot-American chemist known for his research in the area of natural products total synthesis. He is currently Harry C. and Olga K. Wiess Professor of ...
& E. G. Sorensen, summarized by J. Mulzer & D. Riether, and G. W. Craig, besides many other publications where these epochal syntheses are discussed.
The Harvard/ETH approach to the synthesis of cobyric acid: the path to the common corrinoid intermediate via A/B-corrin-ring closure
In the A/B approach to cobyric acid, the Harvard A-D-component was coupled to the ETH B-C-component between rings D and C, and then closed to a corrin between rings A and B. Both these critical steps were accomplished by C,C-coupling via sulfide contraction, a new reaction type developed in the synthesis of the B-C-component at ETH. The A-D-component was synthesized at Harvard from a ring-A precursor (prepared from achiral starting materials), and a ring-D precursor prepared from (−)-camphor. A model A-D-component was used to explore the coupling conditions; this component differed from the A-D-component used in the final synthesis by having as the functional group at the ring-D f-side chain amethyl ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
group (like all other side chains) instead of a nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
group.
The ETH approach to the synthesis of cobyric acid: the path to the common corrinoid intermediate via A/D-corrin-ring closure
In the A/D approach to the synthesis of cobyric acid, the four ring precursors (ring-C precursor only formally so) derive from the two enantiomers of one commonchiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
starting material. All three vinylogous amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
bridges that connect the four peripheral rings were constructed by the sulfide contraction method, with the B-C-component already prepared for the A/B-approach serving as an intermediate. The photochemical A/D-secocorrin→corrin cycloisomerization, by which the corrin ring was closed between rings A and D, is a novel process, targeted and found to exist in a model study ( cf. fig. 2).
ETH/Harvard: the jointly executed final steps from the common corrinoid intermediate to cobyric acid
The final steps from the common corrinoid intermediate E-37/HE-44 to cobyric acid E-44/HE-51 were carried out by the two groups collaboratively and in parallel, theETH
(colloquially)
, former_name = eidgenössische polytechnische Schule
, image = ETHZ.JPG
, image_size =
, established =
, type = Public
, budget = CHF 1.896 billion (2021)
, rector = Günther Dissertori
, president = Joël Mesot
, a ...
group working with material produced by the A/D approach, and the Harvard
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of higher le ...
group with that from the A/B approach. What the two groups in fact accomplished thus were the common final steps of two different syntheses.
The tasks in this end phase of the project were the regioselective introduction of methyl groups at the two meso positions C-5 and C-15 of E-37/HE-44, followed by conversion of all its peripheral carboxyl functions into primary amide groups, excepting that in side chain f at ring D, which had to end up as free carboxyl. These conceptually simple finishing steps turned out to be rather complex in execution, including unforeseen pitfalls like a dramatic loss of precious synthetic material in the so-called "Black Friday" (July 9, 1971).
Notes
References
{{reflist, refs= {{cite journal, doi=10.1002/ange.19630751827, title=Versuche zur Synthese des Vitamins B12, journal=Angewandte Chemie, volume=75, issue=18, pages=871–872, date=1963, last1=Woodward, first1=R. B., bibcode=1963AngCh..75..871W, author-link1=Robert Burns Woodward {{cite book, last1=Woodward, first1=R. B., author-link1=Robert Burns Woodward, chapter=The conservation of orbital symmetry, title=Aromaticity, series=Chemical Society Special Publication, volume=21, publisher=Royal Society of Chemistry, location=London, date=1967, pages=217–249 {{cite journal, doi=10.1351/pac196817030519, doi-access=free, title=Recent advances in the chemistry of natural products, journal=Pure and Applied Chemistry, volume=17, issue=3–4, pages=519–547, date=1968, last1=Woodward, first1=R. B., pmid=5729287, author-link1=Robert Burns Woodward {{cite journal, doi=10.1351/pac197125010283, doi-access=free, title=Recent advances in the chemistry of natural products, journal=Pure and Applied Chemistry, volume=25, pages=283–304, date=1971, last1=Woodward, first1=R. B., issue=1 , pmid=5095424, author-link1=Robert Burns Woodward {{cite AV media, last=Woodward, first=Robert B., others=Introduction by David Dolphin, date=November 27, 1972, title=R.B. Woodward Total Synthesis of Vitamin B12 Lecture - Part 1, type=recorded lecture, language=en, url=https://www.youtube.com/watch?v=YvEB05xdAy4 , archive-url=https://ghostarchive.org/varchive/youtube/20211221/YvEB05xdAy4 , archive-date=2021-12-21 , url-status=live, access-date=2020-01-25, location=Harvard University, Cambridge MA (U.S.A.), publisher=YouTube{{cbignore {{cite AV media, last=Woodward, first=Robert B., date=November 27, 1972, title=R.B. Woodward Total Synthesis of Vitamin B12 Lecture - Part 2, type=recorded lecture, language=en, url=https://www.youtube.com/watch?v=AVi_awjWaP4 , archive-url=https://ghostarchive.org/varchive/youtube/20211221/AVi_awjWaP4 , archive-date=2021-12-21 , url-status=live, access-date=2020-01-25, location=Harvard University, Cambridge MA (U.S.A.), publisher=YouTube{{cbignore {{cite journal, doi=10.1351/pac197333010145, doi-access=free, pmid=4684454, title=The total synthesis of vitamin B12, journal=Pure and Applied Chemistry, volume=33, pages=145–178, date=1973, last1=Woodward, first1=R. B., issue=1, author-link1=Robert Burns Woodward {{cite book, last1=Woodward, first1=R. B., author-link1=Robert Burns Woodward, chapter=Synthetic Vitamin B12, date=1979, pages=37–87, editor1-last=Zagalak, editor1-first=B., editor2-last=Friedrich, editor2-first=W., title=Vitamin B12 (Proceedings of the 3rd European Symposium on Vitamin B12 and Intrinsic Factor, University of Zurich, March 5-8, 1979), publisher=W. de Gruyter, location=Berlin, doi=10.1515/9783111510828-005, isbn=3-11-007668-3 {{cite thesis, last=Kaski, first=B. A., date=1971, title=Studies on Packing in Molecular Crystals, type=PhD, publisher=Harvard University, pages=II-1 {{cite book, editor1-last=Benfey, editor1-first=O. Theodor, editor2-last=Morris, editor2-first=Peter J. T., title=Robert Burns Woodward - Architect and Artist in the World of Molecules, series=History of Modern Chemical Sciences series, year=2001, publisher=Chemical Heritage Foundation, location=Philadelphia, isbn=978-0941901253, issn=1069-2452 {{cite book, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, chapter=Die Synthese von Corrinen, language=de, date=1968, pages=181–214, title=Moderni Sviluppi della Sintesi Organica (X Corso estivo di chimica, Fondazione Donegani, Frascati 25.9.-5.10.1967), publisher=Accademia Nazionale dei Lincei, location=Roma, isbn=8821804054, issn=0515-2216 {{cite journal, title=Current Aspects of Corrinoid Synthesis, journal=Proceedings of the Robert A. Welch Foundation Conference on Chemical Research, volume=12, pages=9–47, year=1968, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, issn=0557-1588, doi=10.3929/ethz-b-000467558, doi-access=free {{cite journal, doi=10.1039/qr9702400366, title=Centenary Lecture (Delivered November 1969). Roads to corrins, journal=Quarterly Reviews, Chemical Society, volume=24, issue=3, pages=366–415, date=1970, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser {{cite book, isbn=0-408-70316-4, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, title=Studies on Organic Synthesis, series=XXIIIrd International Congress of Pure and Applied Chemistry: special lectures presented at Boston, USA, 26-30 July 1971, volume=2, publisher=Butterworths, location=London, date=1971, pages=69–106, doi=10.3929/ethz-a-010165162, doi-access=free, hdl=20.500.11850/84699 {{cite journal, title=Totalsynthese von Vitamin B12: die photochemische Secocorrin-Corrin-Cycloisomerisierung, journal=Chimia, type=abstract of lecture, volume=26, pages=320, year=1972, last1=Fuhrer, first1=W., last2=Schneider, first2=P., last3=Schilling, first3=W., last4=Wild, first4=H., last5=Schreiber, first5=J., last6=Eschenmoser, first6=A., author-link6=Albert Eschenmoser{{cite journal, title=Totalsynthese von Vitamin B12: Endstufen, journal=Chimia, type=abstract of lecture, volume=26, pages=320, year=1972, last1=Maag, first1=H., last2=Obata, first2=N., last3=Holmes, first3=A., last4=Schneider, first4=P., last5=Schilling, first5=W., last6=Schreiber, first6=J., last7=Eschenmoser, first7=A., author-link7=Albert Eschenmoser {{cite journal, doi=10.1002/nadc.19720200804, title="Herr Woodward bedauert, daß die Sache fertig ist." Woodward und Eschenmoser über Vitamin B12 und die Situation der organischen Chemie, journal=Nachrichten aus Chemie und Technik, volume=20, issue=8, pages=147–150, date=2010 {{cite journal, doi=10.1007/BF00606511, pmid=4453344, title=Organische Naturstoffsynthese heute. Vitamin B12 als Beispiel, journal=Die Naturwissenschaften, volume=61, issue=12, pages=513–525, date=1974, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, bibcode=1974NW.....61..513E, s2cid=45688091 {{cite journal, doi=10.1126/science.867037, pmid=867037, title=Natural product synthesis and vitamin B12, journal=Science, volume=196, issue=4297, pages=1410–1420, date=1977, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, last2=Wintner, first2=C., bibcode=1977Sci...196.1410E {{cite book, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, chapter=RBW, Vitamin B12, and the Harvard-ETH Collaboration, date=2001, pages=23–38, editor1-last=Benfey, editor1-first=O. Theodor, editor2-last=Morris, editor2-first=Peter J. T., title=Robert Burns Woodward - Architect and Artist in the World of Molecules, series=History of Modern Chemical Sciences series, publisher=Chemical Heritage Foundation, location=Philadelphia, isbn=978-0941901253, issn=1069-2452 {{cite journal, title=Ein Beispiel zur Anwendung der schnellen Flüssigchromatogarphie in der organischen Synthese, journal=Chimia, volume=25, issue=12, pages=405–407, year=1971, last1=Schreiber, first1=J. {{cite journal, doi=10.1002/anie.196404901, title=A Synthetic Route to the Corrin System, journal=Angewandte Chemie International Edition in English, volume=3, issue=7, pages=490–496, date=1964, last1=Bertele, first1=E., last2=Boos, first2=H., last3=Dunitz, first3=J. D., author-link3=Jack D. Dunitz, last4=Elsinger, first4=F., last5=Eschenmoser, first5=A., author-link5=Albert Eschenmoser, last6=Felner, first6=I., last7=Gribi, first7=H. P., last8=Gschwend, first8=H., last9=Meyer, first9=E. F., last10=Pesaro, first10=M., last11=Scheffold, first11=R. {{cite journal, doi=10.1002/anie.196708643, title=rac.-Dicyano-(1,2,2,7,7,12,12-heptamethylcorrin)-cobalt(III), journal=Angewandte Chemie International Edition in English, volume=6, issue=10, pages=864–866, date=1967, last1=Felner-Caboga, first1=I., last2=Fischli, first2=A., last3=Wick, first3=A., last4=Pesaro, first4=M., last5=Bormann, first5=D., last6=Winnacker, first6=E.L., last7=Eschenmoser, first7=A., author-link7=Albert Eschenmoser {{cite journal, doi=10.1002/anie.196903431, pmid=4977933, title=A New Type of Corrin Synthesis, journal=Angewandte Chemie International Edition in English, volume=8, issue=5, pages=343–348, date=1969, last1=Yamada, first1=Yasuji, last2=Miljkovic, first2=D., last3=Wehrli, first3=P., last4=Golding, first4=B., last5=Löliger, first5=P., last6=Keese, first6=R., last7=Müller, first7=K., last8=Eschenmoser, first8=A., author-link8=Albert Eschenmoser {{cite journal, doi=10.1351/pac196920010001, title=The role of transition metals in the chemical synthesis of corrins, journal= Pure and Applied Chemistry, volume=20, issue=1, pages=1–23, date=1969, last=Eschenmoser, first=A., author-link=Albert Eschenmoser, doi-access=free {{cite book, isbn=978-0471939474, last1=Eschenmoser, first1=A., author-link1=Albert Eschenmoser, chapter=B12: reminiscences and afterthoughts, date=1994, pages=309–336, title=The Biosynthesis of the Tetrapyrrole Pigments, editor1-last=Chadwick, editor1-first=Derek J., editor2-last=Ackrill, editor2-first=Kate, series=Ciba Foundation Symposium 180 (Novartis Foundation Symposia 105), publisher=J. Wiley & Sons, location=Chichester {{cite journal, doi=10.1002/hlca.19710540229, title=Sulfidkontraktion via alkylative Kupplung: Eine Methode zur Darstellung von β-Dicarbonylderivaten. Über synthetische Methoden, 1. Mitteilung, journal=Helvetica Chimica Acta, volume=54, issue=2, pages=710–734, date=1971, last1=Roth, first1=M., last2=Dubs, first2=P., last3=Götschi, first3=E., last4=Eschenmoser, first4=A., author-link4=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.19640470835, title=Claisen'sche Umlagerungen bei Allyl- und Benzylalkoholen mit Hilfe von Acetalen des ''N,N''-Dimethylacetamids. Vorläufige Mitteilung, journal=Helvetica Chimica Acta, volume=47, issue=8, pages=2425–2429, date=1964, last1=Wick, first1=A. E., last2=Felix, first2=Dorothee, last3=Steen, first3=Katharina, last4=Eschenmoser, first4=A., author-link4=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.19690520418, title=Claisen'sche Umlagerungen bei Allyl- und Benzylalkoholen mit 1-Dimethylamino-1-methoxy-äthen, journal=Helvetica Chimica Acta, volume=52, issue=4, pages=1030–1042, date=1969, last1=Felix, first1=Dorothee, last2=Gschwend-Steen, first2=Katharina, last3=Wick, first3=A. E., last4=Eschenmoser, first4=A., author-link4=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.19720550640, title=α-Chlor-nitrone I: Darstellung und Ag+-induzierte Reaktion mit Olefinen. Über synthetische Methoden, 5. (Vorläufige) Mitteilung, journal=Helvetica Chimica Acta, volume=55, issue=6, pages=2187–2198, date=1972, last1=Kempe, first1=U. M., last2=Das Gupta, first2=T. K., last3=Blatt, first3=K., last4=Gygax, first4=P., last5=Felix, first5=Dorothee, last6=Eschenmoser, first6=A., author-link6=Albert Eschenmoser {{cite journal, doi=10.1002/anie.198800051, title=Vitamin B12: Experiments Concerning the Origin of Its Molecular Structure, journal=Angewandte Chemie International Edition in English, volume=27, pages=5–39, date=1988, last1=Eschenmoser, first1=Albert, author-link1=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201400399, title=Introductory Remarks on the Publication Series 'Corrin Syntheses-Parts I-VI', journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1475–1482, date=2015, last1=Eschenmoser, first1=Albert, author-link1=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201400277, doi-access=free, title=Corrin Syntheses. Part I, journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1483–1600, date=2015, last1=Eschenmoser, first1=Albert, author-link1=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201200095, title=Corrin Syntheses. Part II, journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1601–1682, date=2015, last1=Scheffold, first1=Rolf, last2=Bertele, first2=Erhard, last3=Gschwend, first3=Heinz, last4=Häusermann, first4=Werner, last5=Wehrli, first5=Pius, last6=Huber, first6=Willi, last7=Eschenmoser, first7=Albert, author-link7=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201200342, title=Corrin Syntheses. Part IV, journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1755–1844, date=2015, last1=Bertele, first1=Erhard, last2=Scheffold, first2=Rolf, last3=Gschwend, first3=Heinz, last4=Pesaro, first4=Mario, last5=Fischli, first5=Albert, last6=Roth, first6=Martin, last7=Schossig, first7=Jürgen, last8=Eschenmoser, first8=Albert, author-link8=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201200308, title=Corrin Syntheses. Part III, journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1683–1754, date=2015, last1=Pesaro, first1=Mario, last2=Elsinger, first2=Fritz, last3=Boos, first3=Helmut, last4=Felner-Caboga, first4=Ivo, last5=Gribi, first5=Hanspeter, last6=Wick, first6=Alexander, last7=Gschwend, first7=Heinz, last8=Eschenmoser, first8=Albert, author-link8=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201300064, title=Corrin Syntheses. Part V, journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1845–1920, date=2015, last1=Blaser, first1=Hans-Ulrich, last2=Winnacker, first2=Ernst-Ludwig, author-link2=Ernst-Ludwig Winnacker, last3=Fischli, first3=Albert, last4=Hardegger, first4=Bruno, last5=Bormann, first5=Dieter, last6=Hashimoto, first6=Naoto, last7=Schossig, first7=Jürgen, last8=Keese, first8=Reinhart, last9=Eschenmoser, first9=Albert, author-link9=Albert Eschenmoser {{cite journal, doi=10.1002/hlca.201500012, doi-access=free, title=Corrin Syntheses. Part VI, journal=Helvetica Chimica Acta, volume=98, issue=11–12, pages=1921–2054, date=2015, last1=Yamada, first1=Yasuji, last2=Wehrli, first2=Pius, last3=Miljkovic, first3=Dusan, last4=Wild, first4=Hans-Jakob, last5=Bühler, first5=Niklaus, last6=Götschi, first6=Erwin, last7=Golding, first7=Bernard, last8=Löliger, first8=Peter, last9=Gleason, first9=John, last10=Pace, first10=Brian, last11=Ellis, first11=Larry, last12=Hunkeler, first12=Walter, last13=Schneider, first13=Peter, last14=Fuhrer, first14=Walter, last15=Nordmann, first15=René, last16=Srinivasachar, first16=Kasturi, last17=Keese, first17=Reinhart, last18=Müller, first18=Klaus, last19=Neier, first19=Reinhard, last20=Eschenmoser, first20=Albert, author-link20=Albert Eschenmoser {{cite thesis, type=PhD, title=Synthetische Versuche in Richtung auf natürlich vorkommende Corrinoide, last1=Wild, first1=Jost, hdl=20.500.11850/132003, date=1964, publisher=ETH Zürich (Promotionsarbeit Nr. 3492), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/132003/eth-20102-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000088927, doi-access=free {{cite thesis, type=PhD, title=Darstellung eines Zwischenproduktes zur Synthese von Vitamin B12, last1=Locher, first1=Urs, hdl=20.500.11850/131398, date=1964, publisher=ETH Zürich (Promotionsarbeit Nr. 3611), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/131398/eth-20032-02.pdf?sequence=2&isAllowed=y, doi=10.3929/ethz-a-000090323, doi-access=free {{cite thesis, type=PhD, title=Untersuchungen in Richtung einer Totalsynthese von Vitamin B12, last1=Wick, first1=Alexander, hdl=20.500.11850/132537, date=1964, publisher=ETH Zürich (Promotionsarbeit Nr. 3617), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/132537/eth-20219-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000090041, doi-access=free {{cite thesis, type=PhD, title=Darstellung eines die Ringe B und C umfassenden Zwischenproduktes zur Synthese von Vitamin B12, last1=Löliger, first1=Peter, hdl=20.500.11850/133844, date=1968, publisher=ETH Zürich (Promotionsarbeit Nr. 4074), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/133844/eth-20827-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000093406, doi-access=free {{cite thesis, type=PhD, date=1968, last1=Fischli, first1=Albert, title=Die Synthese metallfreier Corrine, hdl=20.500.11850/137445, publisher=ETH Zürich (Promotionsarbeit Nr. 4077), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/137445/eth-21903-02.pdf?sequence=2&isAllowed=y, doi=10.3929/ethz-a-000267791, doi-access=free {{cite thesis, type=PhD, date=1968, last1=Werthemann, first1=Lucius, title=Untersuchungen an Kobalt(II)- und Kobalt(III)-Komplexen des Cobyrinsäure-heptamethylesters, hdl=20.500.11850/133926, publisher=ETH Zürich (Promotionsarbeit Nr. 4097), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/133926/eth-20834-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000093488, doi-access=free {{cite thesis, type=PhD, title=Ligandreaktivität synthetischer Kobalt(III)-Corrin-Komplexe, last1=Winnacker, first1=Ernst-Ludwig, author-link1=Ernst-Ludwig Winnacker , hdl=20.500.11850/136417, date=1968, publisher=ETH Zürich (Promotionsarbeit Nr. 4177), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/136417/eth-21820-02.pdf?sequence=2&isAllowed=y, doi=10.3929/ethz-a-000150375, doi-access=free {{cite thesis, type=PhD, title=Darstellung von Zwischenprodukten zur Synthese von Vitamin B12, last1=Wiederkehr, first1=René, hdl=20.500.11850/131502, date=1968, publisher=ETH Zürich (Promotionsarbeit Nr. 4239), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/131502/eth-20036-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000087656, doi-access=free {{cite thesis, type=PhD, title=Beiträge zur Synthese von Vitamin B12: Darstellung vinyloger Amidine mit der Sulfidkontraktions-Methode, last1=Dubs, first1=Paul, hdl=20.500.11850/133822, year=1969, publisher=ETH Zürich (Promotionsarbeit Nr. 4297), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/133822/eth-20826-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000093384, doi-access=free {{cite thesis, type=PhD, title=Beiträge zur Synthese von Vitamin B12: Zum Problem der (C-D)-Verknüpfung, last1=Huber, first1=Willy, hdl=20.500.11850/132700, date=1969, publisher=ETH Zürich (Promotionsarbeit Nr. 4298), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/132700/eth-20237-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000090323, doi-access=free {{cite thesis, type=PhD, date=1971, last1=Blaser, first1=Hans-Ulrich, title=Herstellung und Eigenschaften eines metallfreien Corrin-Derivates, hdl=20.500.11850/133210, publisher=ETH Zürich (Promotionsarbeit Nr. 4662), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/133210/eth-20506-02.pdf?sequence=2&isAllowed=y, doi=10.3929/ethz-a-000091385, doi-access=free {{cite thesis, type=PhD, title=Totalsynthese von Derivaten des Dicyano-cobalt(III)-5,15-bis-nor-cobyrinsäure-hepta-methylesters, last1=Schneider, first1=Peter, hdl=20.500.11850/132893, date=1972, publisher=ETH Zürich (Promotionsarbeit Nr. 4819), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/132893/eth-20315-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000090603, doi-access=free {{cite thesis, type=PhD, title=Die Synthese von Corrin-Komplexen durch photochemische A/D-Cycloisomerisierung, last1=Wild, first1=Hans-Jakob, hdl=20.500.11850/132648, date=1972, publisher=ETH Zürich (Promotionsarbeit Nr. 4848), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/132648/eth-20228-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000090212, doi-access=free {{cite thesis, type=PhD, title=Totalsynthese von Vitamin B12: Der photochemische Weg, last1=Fuhrer, first1=Walter, hdl=20.500.11850/131362, date=1973, publisher=ETH Zürich (Promotionsarbeit Nr. 5158), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/131362/eth-20030-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000086601, doi-access=free {{cite thesis, type=PhD, title=Totalsynthese von Vitamin B12: Dicyano-Co(III)-Cobyrinsäure-Hexamethylester-f-Amid, last1=Maag, first1=Hans, hdl=20.500.11850/131110, date=1973, publisher=ETH Zürich (Promotionsarbeit Nr. 5173), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/131110/eth-20020-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000085446, doi-access=free {{cite thesis, type=PhD, date=1974, last1=Schilling, first1=Walter, title=Totalsynthese von Vitamin B12. Darstellung von Zwischenprodukten und partialsynthetische Endstufen, hdl=20.500.11850/131064, publisher=ETH Zürich (Promotionsarbeit Nr. 5352), url=https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/131064/eth-20016-01.pdf?sequence=1&isAllowed=y, doi=10.3929/ethz-a-000085344, doi-access=free {{cite journal, doi=10.1038/178064a0, pmid=13348621, title=Structure of Vitamin B12, journal=Nature, volume=178, issue=4524, pages=64–66, date=1956, last1=Hodgkin, first1=Dorothy Crowfoot, author-link1=Dorothy Hodgkin, last2=Kamper, first2=Jennifer, last3=MacKay, first3=Maureen, last4=Pickworth, first4=Jenny, author-link4=Jenny Glusker, last5=Trueblood, first5=Kenneth N., author-link5=Kenneth Nyitray Trueblood, last6=White, first6=John G., bibcode=1956Natur.178...64H, s2cid=4210164 {{cite journal, doi=10.1098/rspa.1964.0042, title=The structure of Vitamin B12 VI. The structure of crystals of vitamin B12 grown from and immersed in water, journal=Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences, year=1964, volume=278, issue=1372, pages=1–26, bibcode=1964RSPSA.278....1B, last1=Brink-Shoemaker, first1=Clara, last2=Cruickshank, first2=D. W. J., last3=Crowfoot Hodgkin, first3=Dorothy, author-link3=Dorothy Hodgkin, last4=Kamper, first4=M. Jennifer, last5=Pilling, first5=Diana, s2cid=93447375 {{cite web, url=https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1284686&DatabaseToSearch=Published, title=CSD Entry: VITAMB Vitamin B12 hydrate, publisher=Cambridge Structural Database
The Cambridge Structural Database (CSD) is both a repository and a validated and curated resource for the three-dimensional structural data of molecules generally containing at least carbon and hydrogen, comprising a wide range of organic compound ...
CCDC/Fiz Karlsruhe, access-date=2020-12-24
{{cite journal, title=Vitamin B12 and the B12 Coenzymes, journal=Vitamins and Hormones, volume=50, pages=1–76, year=1995, last1=Glusker, first1=Jenny P., doi=10.1016/S0083-6729(08)60654-8, pmid=7709599, isbn=9780127098500, author-link1=Jenny Glusker
{{cite journal, doi=10.1515/znb-1960-0522, title=Notizen: Vitamin B12-Faktor V1a, ein neuer "inkompletter" Grundkörper der Vitamin B12-Gruppe, journal=Zeitschrift für Naturforschung B, volume=15, issue=5, pages=336–337, date=1960, last1=Bernhauer, first1=K., last2=Dellweg, first2=H., last3=Friedrich, first3=W., last4=Gross, first4=Gisela, last5=Wagner, first5=F., s2cid=98606543
{{cite journal, title=5-Nor-, 15-Nor- und 5,15-Dinorcorrinoide, journal=Hoppe-Seyler's Zeitschrift für physiologische Chemie, volume=354, issue=8, pages=957–966, year=1973, last1=Jauernig, first1=D., last2=Rapp, first2=P., last3=Ruoff, first3=G., doi=10.1515/bchm2.1973.354.2.957
{{cite journal, doi=10.1039/j39710003736, pmid=5167083, title=Cyano-13-epicobalamin (Neovitamin B12) and its relatives, journal=Journal of the Chemical Society C: Organic, volume=22, pages=3736–43, year=1971, last1=Bonnett, first1=R., last2=Godfrey, first2=J. M., last3=Math, first3=V. B.
{{cite journal, doi=10.1039/DT9740000820, title=Luminescence properties of some synthetic metallocorrins, journal=Journal of the Chemical Society, Dalton Transactions, issue=8, pages=820–828, date=1974, last1=Gardiner, first1=Maureen, last2=Thomson, first2=Andrew J.
{{cite journal, doi=10.1002/jlac.199619960306, title=Preparation and Structure Proof of the Four Isomeric Dicyanocobyrinic Acid Hexamethyl Ester Monoamides Carrying the Amide Group on a Propionic Acid Side Chain, journal=Liebigs Annalen, volume=1996, issue=3, pages=323–326, date=2006, last1=Ernst, first1=Ludger, last2=Maag, first2=Hans
{{cite journal, doi=10.1002/cber.190203503216, title=Reactionen des Campherchinons, journal=Berichte der Deutschen Chemischen Gesellschaft, volume=35, issue=3, pages=3829–3843, date=1902, last1=Manasse, first1=O., last2=Samuel, first2=E., url=https://zenodo.org/record/1426060
{{cite journal, doi=10.1039/np9850200253, pmid=3906448, title=Camphor: A chiral starting material in natural product synthesis, journal=Natural Product Reports, volume=2, issue=3, pages=253–289, date=1985, last1=Money, first1=T.
{{cite journal, doi=10.1021/ja01578a049, title=The synthesis of α-santalene and of trans-Δ11,12-iso-α-santalene, journal=Journal of the American Chemical Society, volume=79, issue=21, pages=5773–5777, date=1957, last1=Corey, first1=E. J., author-link1=Elias James Corey, last2=Chow, first2=S. W., last3=Scherrer, first3=R. A.
{{cite journal, title=Santalol series. II. Synthesis of d- and dl-π-hydroxycamphor, d- and dl-teresantalol, and d- and dl-tricycloekasantalic acid, journal=Journal of the Indian Chemical Society, volume=21, pages=271–280, date=1944, last1=Guha, first1=P. C., last2=Bhattachargya, first2=S. C.
{{cite journal, doi=10.1021/ja01532a048, title=Acid-catalyzed cleavage of π-substituted tricyclenes. Synthesis of 3,8-cyclocamphor, journal=Journal of the American Chemical Society, volume=81, issue=23, pages=6305–6309, date=1959, last1=Corey, first1=E. J., author-link1=Elias James Corey, last2=Ohno, first2=Masaji, last3=Chow, first3=S. W., last4=Scherrer, first4=Robert A.
{{cite journal, doi=10.1021/ja01354a043, title=Studies on π-camphor derivatives. II. The identity of dihydro-teresantalic acid with 7-π-apocamphan-carboxylic acid, journal=Journal of the American Chemical Society, volume=53, issue=3, pages=1097–1103, date=1931, last1=Hasselstrom, first1=Torsten
{{cite journal, doi=10.1093/chromsci/7.2.85, title=High Efficiency, High Speed Liquid Chromatography in Columns, journal=Journal of Chromatographic Science, volume=7, issue=2, pages=85–90, date=1969, last1=Huber, first1=J. F. K.
{{cite journal, doi=10.1021/ja00706a074, title=Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene, journal=Journal of the American Chemical Society, volume=92, issue=3, pages=741–743, date=1970, last1=Johnson, first1=William Summer, author-link1=William Summer Johnson, last2=Werthemann, first2=Lucius, last3=Bartlett, first3=William R., last4=Brocksom, first4=Timothy J., last5=Li, first5=Tsung-Tee, last6=Faulkner, first6=D. John, last7=Petersen, first7=Michael R.
{{cite journal, doi=10.1021/ja00426a033, title=The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation, journal=Journal of the American Chemical Society, volume=98, issue=10, pages=2868–2877, date=1976, last1=Ireland, first1=Robert E., last2=Mueller, first2=Richard H., last3=Willard, first3=Alvin K.
{{cite journal, title=Vitamin B12: the struggle toward synthesis, journal=Chemical & Engineering News, volume=51, issue=11/March 12, pages=16–29, year=1973, last1=Krieger, first1=J. H., doi=10.1021/cen-v051n011.p016
{{cite journal, title=Utilisation de la chromatographie liquide haute pression en synthese organique, journal=Informations Chimique, volume=119, pages=229–231, year=1973, last1=Hertzog, first1=D.
{{cite journal, doi=10.2533/000942906777675029, doi-access=free, title=Recollecting the Institute of Organic Chemistry, ETH Zürich, 1972-1990, journal=Chimia, volume=60, issue=3, pages=142–148, year=2006, last1=Wintner, first1=Claude E.
{{cite book, last1=Goto, first1=Toshio, chapter=chapter 11.34: Synthesis of vitamin B12, date=1975, pages=480–496, editor1-last=Nakanishi, editor1-first=Koji, editor1-link=Koji Nakanishi, editor2-last=Goto, editor2-first=Toshio, editor3-last=Sho, editor3-first=Ito, editor4-last=Natori, editor4-first=Shinsaku, editor5-last=Nozoe, editor5-first=Shigeo, title=Natural Products Chemistry, volume=2, publisher=Kodansha/Academic Press, location=Tokyo, isbn=0-12-513902-0
{{cite book, isbn=978-0-471-03655-5, last1=Stevens, first1=R. V., chapter=The Total Synthesis of Vitamin B12, date=1982, pages=169–200, title=Vitamin B12, volume=1, editor-last1=Dolphin, editor-first1=D., editor1-link=David Dolphin, publisher=John Wiley & Sons, location=New York
{{cite book, last1=Jackson, first1=A. H., last2=Smith, first2=K. M., chapter=The Total Synthesis of Pyrrole Pigments, editor-last=Apsimon, editor-first=John, title=Total Synthesis of Natural Products, date=1973, volume=1, pages=143–278, doi=10.1002/9780470129647.ch3, isbn=9780471032519
{{cite book, isbn=978-3-527-29231-8, last1=Nicolaou, first1=K. C., author-link1=K. C. Nicolaou, last2=Sorensen, first2=E. J., chapter=Chapter 8: Vitamin B12. R. B. Woodward and A. Eschenmoser (1973), date=1996, page99
136, title=Classics in Total Synthesis: Targets, Strategies, Methods, url=https://archive.org/details/classicstotalmet00kcni_087, url-access=limited, publisher=VCH Verlag Chemie, location=Weinheim {{cite journal, doi=10.1021/ed075p1225, title=The Art and Science of Organic and Natural Products Synthesis, journal=Journal of Chemical Education, volume=75, issue=10, pages=1225–1258, date=1998, last1=Nicolaou, first1=K. C., author-link1=K. C. Nicolaou, last2=Sorensen, first2=E. J., last3=Winssinger, first3=N., bibcode=1998JChEd..75.1225N, doi-access=free {{cite journal, doi=10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L, pmid=10649349, title=The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century, journal=Angewandte Chemie International Edition, volume=39, issue=1, pages=44–122, date=2000, last1=Nicolaou, first1=K. C., author-link1=K. C. Nicolaou, last2=Vourloumis, first2=Dionisios, last3=Winssinger, first3=Nicolas, last4=Baran, first4=Phil S., author-link4=Phil S. Baran {{cite journal, doi=10.1002/1099-0690(200301)2003:1<30::AID-EJOC30>3.0.CO;2-I, title=Total Synthesis of Cobyric Acid: Historical Development and Recent Synthetic Innovations, journal=European Journal of Organic Chemistry, volume=2003, pages=30–45, date=2003, last1=Riether, first1=Doris, last2=Mulzer, first2=Johann, author-link2=Johann Mulzer {{cite journal, doi=10.1007/s12045-014-0064-4, title=Eschenmoser approach to vitamin B12 by A/D strategy, journal=Resonance, volume=19, issue=7, pages=624–640, date=2014, last1=Craig, first1=G. Wayne, s2cid=118161709 {{cite journal, doi=10.1142/S1088424615500960, title=Total synthesis of vitamin B12 - a fellowship of the ring, journal=Journal of Porphyrins and Phthalocyanines, volume=20, pages=1–20, date=2016, last1=Craig, first1=G. Wayne {{cite journal, doi=10.1126/science.1067545, pmid=11729290, title=Natural product synthesis: the art of total synthesis, journal=Science, volume=294, issue=5548, pages=1842–1843, date=2001, last1=Marko, first1=I. E., s2cid=22467000 {{cite journal, doi=10.1039/qr9712500031, title=Recent developments in the chemistry of pyrrolic compounds, journal=Quarterly Reviews, Chemical Society, volume=25, pages=31–85, date=1971, last1=Smith, first1=K. M. {{cite book, doi=10.1142/9789814397605_0020, last1=Montforts, first1=Franz-Peter, last2=Osmers, first2=Martina, last3=Leupold, first3=Dennis, chapter=Chemical Synthesis of Artificial Corrins, editor1-last=Kadish, editor1-first=Karl M., editor2-last=Smith, editor2-first=Kevin M., editor3-last=Guilard, editor3-first=Roger, title=Handbook of Porphyrin Science, volume=25, date=2012, pages=265–307, publisher=World Scientific Publishing, isbn=978-981-4397-66-7 {{cite web, url=https://www.research-collection.ethz.ch/, title=ETH Research Collection (previously ETH e-collection), publisher=
ETH Zurich
(colloquially)
, former_name = eidgenössische polytechnische Schule
, image = ETHZ.JPG
, image_size =
, established =
, type = Public
, budget = CHF 1.896 billion (2021)
, rector = Günther Dissertori
, president = Joël Mesot
, ac ...
, access-date=2020-01-25
{{cite web, url=https://de.slideshare.net/EngelbertZass1/of-a-landmark-total-synthesis-yet-unpublished-in-full-experimental-detail-vitamin-b12, last=Zass, first=E., title=Of a Landmark Total Synthesis yet Unpublished in Full Experimental Detail - Vitamin B12 (Slides of the Skolnik Award Lecture at the 248th ACS National Meeting, San Francisco CA, Aug. 12, 2014), date=2014, website=SlideShare, publisher=LinkedIn, access-date=2020-01-25 See also {{cite journal, title=Herman Skolnik Award Symposium Honoring Engelbert Zass, journal=Chemical Information Bulletin, volume=66, issue=4/Winter 2014, pages=37–40, year=2014, last=Warr, first=Wendy, url=https://bulletin.acscinf.org/node/665, access-date=2020-01-25
Total synthesis
Organocobalt compounds
Vitamin B12