Unsaturated hydrocarbon on:
[Wikipedia]
[Google]
[Amazon]
300px, Structure of an ethene molecule, the simplest unsaturated hydrocarbon
Unsaturated hydrocarbons are
Cis-2-Buten.svg, structure of cis-2-butene
Trans-2-Buten.svg, structure of trans-2-butene
Trans-2-butene.svg, (''E'')-But-2-ene
Cis-2-butene.svg, (''Z'')-But-2-ene
The cis- and trans- configuration requires the existence of a carbon chain or that at least one
 Just like their
Just like their
 Compared to the single σ C−C bonds in the saturated hydrocarbons, the unsaturated ones have electron density in the π bonds, which do not have much electron density overlapping as the σ. As a result, the
Compared to the single σ C−C bonds in the saturated hydrocarbons, the unsaturated ones have electron density in the π bonds, which do not have much electron density overlapping as the σ. As a result, the
 The reaction equation for bromine addition of
The reaction equation for bromine addition of
 The hydrohalogenation involves addition of H−X to unsaturated hydrocarbons. This will decrease one π C=C bond and result in 2 C−H and C−X σ bonds with 2 separate carbons. The formation of the intermediate carbocation is selective and follows the
The hydrohalogenation involves addition of H−X to unsaturated hydrocarbons. This will decrease one π C=C bond and result in 2 C−H and C−X σ bonds with 2 separate carbons. The formation of the intermediate carbocation is selective and follows the
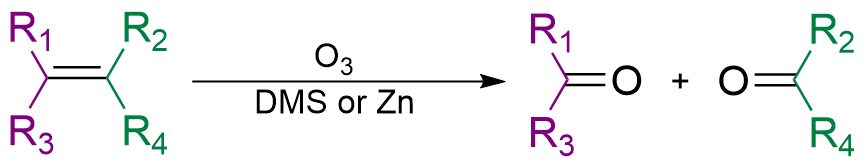
 For unsaturated hydrocarbons, ring structure and π bonds can both increase the degree of unsaturation, interchange between ring structure and π bonds may occur under special conditions. For instance, for a
For unsaturated hydrocarbons, ring structure and π bonds can both increase the degree of unsaturation, interchange between ring structure and π bonds may occur under special conditions. For instance, for a
 The delocalized π bond in unsaturated hydrocarbons provide high electron density, making the molecule possible to become a metal
The delocalized π bond in unsaturated hydrocarbons provide high electron density, making the molecule possible to become a metal
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
s that have double or triple covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s between adjacent carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s. The term "unsaturated" means more hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
atoms may be added to the hydrocarbon to make it saturated (i.e. consisting all single bonds). The configuration of an unsaturated carbons include straight chain, such as alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, as well as branched chains and aromatic compounds
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
.
Except for aromatic compounds
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
, unsaturated hydrocarbons are mostly reactive and undergo multiple reactions to their multiple bonds.
Nomenclature
For the sake of clearer communication and less misunderstanding, a consistent naming system is necessary, which gives rise to the IUPAC nomenclature. Some standard steps to follow when naming unsaturated hydrocarbon molecules with IUPAC nomenclature are elaborated below. *1. Find and count the number of carbon atoms in the longest carbon chain and use the corresponding number prefix. For example, if the longest carbon chain contains three carbon atoms, use prefix “prop-”. The prefix of number of carbons from 1 to 10 is summarized in the table below. *2. Determine thesuffix
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns, adjectives, and verb endings, which form the conjugation of verbs. Suffixes can carry ...
based on the type of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
.
**If one or more double bonds are present, use suffix “-ene
The suffix -ene is used in organic chemistry to form names of organic compounds where the -C=C- group has been attributed the highest priority according to the rules of organic nomenclature. Sometimes a number between hyphens is inserted before i ...
”.
**If one or more triple bonds are present, use suffix “-yne
In chemistry, the suffix -yne is used to denote the presence of a triple bond.
The suffix follows IUPAC nomenclature, and is mainly used in organic chemistry. However, inorganic compounds featuring unsaturation in the form of triple bonds may ...
”.
**If both double bonds and triple bonds are present, use both suffixes “-ene” and “-yne”. “-ene” usually goes before “-yne” as “e” is prior to “y” lexicographically.
*3. Count the number of double bonds or triple bonds and indicate that by a number prefix before “-ene” or “-yne”. For example, a carbon chain with 4 carbon atoms containing 2 double bonds will be named as “butadiene”.
*4. Add numbers between prefix of number of carbons and “-ene” or “-yne” to indicate the position of starting carbon of double bonds or triple bonds. For example, a carbon chain with 4 carbon atoms containing a double bond between the second carbon and the third carbon will be named as “but-2-ene”.
*5. Lastly, use prefix before the prefix of number of carbons to indicate any side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a l ...
s present. A straight carbon side chain is named simply by adding “-yl” after the prefix representing the number of carbon atoms in that chain. For example, if an ethyl group is attached to the second carbon in pent-2-ene, the molecule will be named as “2-ethylpent-2-ene”. For the naming of more complicated side chain, consult IUPAC nomenclature of organic chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the ''Nomenclature of Or ...
. The side chain prefixes are added to the final name lexicographically, meaning an ethyl group will appear earlier than a methyl group.
**If the compound is circular, use prefix “ cyclo-”. For example, a carbon ring with 5 carbon atoms containing 1 double bond will be named as “cyclopentene”.
Structure
Isomerism
Inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, cis- and trans- prefix
A prefix is an affix which is placed before the Word stem, stem of a word. Adding it to the beginning of one word changes it into another word. For example, when the prefix ''un-'' is added to the word ''happy'', it creates the word ''unhappy'' ...
es are used to describe the position of functional groups attached to carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atoms in a double bond. In Latin, cis and trans mean "on this side of" and "on the other side of" respectively. Therefore, if the functional groups are on the same side of the carbon chain, the bond is assigned cis- configuration, otherwise (i.e. the functional groups are on the opposite side of the carbon chain), the bond is assigned trans- configuration.
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
attached to each carbon is the same. E- and Z- configuration can be used instead in a more general case where all four functional groups attached to carbon atoms in a double bond are different. E- and Z- are the abbreviation
An abbreviation (from Latin ''brevis'', meaning ''short'') is a shortened form of a word or phrase, by any method. It may consist of a group of letters or words taken from the full version of the word or phrase; for example, the word ''abbrevia ...
s of German words ''zusammen'' (together) and ''entgegen'' (opposite). In E- and Z- isomerism, each functional group is assigned a priority based on the Cahn–Ingold–Prelog priority rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named for R.S. Cahn, C.K. Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally name a stereoisomer of a ...
. If the two groups with higher priority are on the same side of the double bond, the bond is assigned Z- configuration, otherwise (i.e. the two groups with higher priority are on the opposite side of the double bond), the bond is assigned E- configuration. Note that cis- and trans- configuration does not have a fixed relationship with E- and Z- configuration.
Orbital hybridization
Carbon is known to have electron configuration of 1s2 2s2 2p2. As the only unpaired electrons it has are the two in the 2p orbitals, carbon is theoretically only capable of forming 2 single bonds. However, this is definitely not true as in reality, each carbon in ethene forms 2 single bonds and 1 double bond whereas each carbon in ethyne forms 1 single bond and 1 triple bond. In fact, it is the orbital hybridization that gives rise to this strange phenomenon. In ethyne like molecules where carbon forms 1 triple bond and 1 single bond, the carbon atom undergoes sp hybridization, meaning the 2s orbital and one 2p orbital are combined to form two sp orbitals, and the other two 2p orbitals left remain unchanged. The angle between the two sp orbitals is 180°, and the first unchanged 2p orbital is perpendicular to the two sp orbitals while the second unchanged 2p orbital is perpendicular to both the two sp orbitals and the first unchanged 2p orbital. The 4 electrons from 2s and 2p orbitals are distributed equally among the two sp orbitals and two 2p orbitals (i.e. one electron in each orbital). During bond formation, one sp orbital from carbon forms a single σ bond with one other atom, and at the same time, the remaining one sp orbital and two 2p orbitals form a σ bond as well as two π bonds (a triple bond) with another atom, resulting in a linear molecular geometry. In ethene like molecules where carbon form 1 double bond and 2 single bonds, the carbon atom undergoes sp2 hybridization, meaning the 2s orbital and two 2p orbitals are combined to form three sp2 orbitals, and the one 2p orbital left remains unchanged. The three sp2 orbitals are in the same plane with a 60° angle between each two of them, and the unchanged 2p orbital is perpendicular to all three sp2 orbitals. The 4 electrons from 2s and 2p orbitals are distributed equally among the three sp2 orbitals and the unchanged 2p orbital (i.e. one electron in each orbital). During bond formation, two sp2 orbitals from carbon form two separate single σ bonds with two other atoms respectively, and at the same time, the remaining one sp orbital and the unchanged 2p orbital form a σ bond as well as a π bond (a double bond) with another atom, resulting in a trigonal planar molecular geometry. There is also sp3 hybridization where the 2s orbital and all three 2p orbitals are combined to form four sp3 orbitals. A carbon with sp3 hybridization will have tetrahedral molecular geometry and is therefore saturated.Degree of unsaturation
Degree of unsaturation is a calculation used to measure the number of π bonds in an unsaturatedorganic molecule
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
. In a common compound composed of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, and halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
, the degree of unsaturation formula can be expressed in the following way:
*DU =
**C = number of carbon atoms in the compound
**N = number of nitrogen atoms in the compound
**F = number of halogen atoms in the compound
**H = number of hydrogen atoms in the compound
*the number of oxygen atoms or any other divalent atoms does not contribute to the degree of unsaturation
The degree of unsaturation also stands for that at most 2×DU hydrogen atoms can be added to the compound to make it saturated.
Physical properties
Boiling and melting point
This is a list showing the boiling points and melting points of saturated and unsaturated hydrocarbons with same number of carbons.saturated
Saturation, saturated, unsaturation or unsaturated may refer to:
Chemistry
* Saturation, a property of organic compounds referring to carbon-carbon bonds
** Saturated and unsaturated compounds
**Degree of unsaturation
** Saturated fat or fatty ac ...
counterparts, the unsaturated hydrocarbons are usually non-polar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more pola ...
. This means the intermolecular forces between unsaturated hydrocarbon molecules are dominantly weak Van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
. The boiling point and melting point of unsaturated hydrocarbons are usually similar as their saturated counterparts with same number of carbon.
The melting and boiling points of unsaturated hydrocarbons compared to saturated ones are determined by two opposing factors. On the one hand, the strength of Van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
depends on the number of electrons in a molecule. Unsaturated hydrocarbons have less electrons than saturated ones, so the boiling and melting point may decrease as intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
decreases. On the other hand, the delocalized π electrons existing in the unsaturated hydrocarbons make the electron flow more easily within one molecule, so temporary dipoles are easier to form. Thus, the Van der Waals force may also increase due to delocalization of electrons. It turns out that alkynes are more affected by electron delocalization and usually have higher boiling points than alkanes with the same number of carbon. Alkenes are more affected by number of electrons and have lower boiling points than alkanes.
The boiling and melting points also depend on the stereochemistry. The cis
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonome ...
alkenes, due to their U-bending shape, cannot arrange themselves as closely as the trans ones, so they will have lower boiling and melting points.
For longer chains of unsaturated hydrocarbons, the effects above still apply. In longer chains, the stereochemical "zig-zag" effect of unsaturated hydrocarbons become the dominant effect, so unsaturated long chain hydrocarbons usually have lower boiling and melting points. The melting point difference between saturated and unsaturated fat
In nutrition science, nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such chemical compound, compounds, most commonly those that occur in living beings or in food.
The term often refers spec ...
inside human body also leads to health issues.
Solubility
Unsaturated hydrocarbons are also non-polar which makes them have low solubility in water. They are easier to dissolve in non-polar organic solvents such asbenzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
.
Spectroscopic Properties
Compared tosaturated hydrocarbons
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whic ...
, the unsaturated hydrocarbons not only contains the C−C bonds and C−H bonds, but also have C=C double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s and C≡C triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
s. As a result, the spectrum will also contain characteristics of these π bondings. Similar as alkanes, the spectroscopy of unsaturated hydrocarbons will not shows the characteristics of other functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s such as alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
(−OH) and carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
(−COOH).
Infrared Spectroscopy
The stretching of C=C bond will give an IR absorption peak at 1670–1600 cm−1, while the bending of C=C bond absorbs between 1000 and 650 cm−1 wavelength. The stretching of C≡C bond absorbs 2100–2140 cm−1(monosubstituted) and 2190–2260 cm−1(disubstituted). The strength of these absorption peaks varies with the place and number of the double or triple bonds. Because of the delocalized π electrons inaromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
groups, the bending of C=C bond in these groups usually absorbs between 1500 and 1700 cm−1.
At the mean time, the absorption peaks of C–H and C–C bond, which are shared with the saturated hydrocarbons, also shows in the IR spectrum of unsaturated hydrocarbons.
NMR Spectroscopy
In 1HNMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
spectroscopy, the hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
bonded to the carbon adjacent to double bonds will give a δH of 4.5–6.5 ppm. The double bond will also deshield the hydrogen attached to the carbons adjacent to sp2 carbons, and this generates δH=1.6–2. ppm peaks. Aromatic groups will have δH=6.5–8.5 ppm peaks. Since the π bondings will make cis/trans isomers, the unsaturated hydrocarbon isomers will appear differently due to different J-coupling
In nuclear chemistry and nuclear physics, ''J''-couplings (also called spin-spin coupling or indirect dipole–dipole coupling) are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that a ...
effect. Cis vicinal hydrogens will have coupling constants in the range of 6–14 Hz, whereas the trans will have coupling constants of 11–18 Hz.
In 13C NMR spectroscopy, compared to the saturated hydrocarbons, the double and triple bonds also deshiled the carbons, making them have low field shift. C=C double bonds usually have chemical shift of about 100–170 ppm.
Chemical Properties
Combustion
Like most otherhydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
s, unsaturated hydrocarbons can go under combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
reactions that produces carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
in complete combustion. The reaction equation is:
*CxHy + O2 → H2O + xCO2
In the absence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, the combustion will turn into incomplete combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
and produce carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
.
The unsaturated hydrocarbons will produce incomplete combustion product more easily than saturated ones. As a result, the combustion of unsaturated hydrocarbons usually have yellow flame
A flame (from Latin ''flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they ...
, different from the blue flame of the saturated ones. This indicates unsaturated hydrocarbon combustion will involve multi-step mechanisms, and the burning of carbon gives the yellow flame color.
Since unsaturated hydrocarbons have less hydrogen content, it will produce less water and decrease the flame moisture
Moisture is the presence of a liquid, especially water, often in trace amounts. Small amounts of water may be found, for example, in the air (humidity), in foods, and in some commercial products. Moisture also refers to the amount of water vapo ...
, as well as decrease the oxygen use. Acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
(ethyne
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
), for example, can be used as fuel.
 Compared to the single σ C−C bonds in the saturated hydrocarbons, the unsaturated ones have electron density in the π bonds, which do not have much electron density overlapping as the σ. As a result, the
Compared to the single σ C−C bonds in the saturated hydrocarbons, the unsaturated ones have electron density in the π bonds, which do not have much electron density overlapping as the σ. As a result, the chemical energy
Chemical energy is the energy of chemical substances that is released when they undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (2018). "How ...
stored in one double bond is less than in two single bonds. Thus, the combustion of unsaturated hydrocarbons, which breaks the carbon–carbon bonds to release energy, release less energy than burning same molarity
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solut ...
of saturated ones with same number of carbons.
This trend can be clearly seen in the list of standard enthalpy of combustion of hydrocarbons.
Electrophilic Addition
The double or triple bonds that must present in unsaturated hydrocarbons provide highelectron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
that make the molecules become perfect spots for electrophilic addition reactions. In this kind of reaction, one π bond between carbons will break into 2 separate σ bonds between each carbon and the added group. A carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
intermediate is usually involved in the mechanism.
Hydrogenation
Hydrogenation is the electrophilic addition ofhydrogen gas
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, a ...
to unsaturated hydrocarbon. The result will be a more saturated hydrocarbon, but not necessarily become a saturated one. For instance, semihydrogenation of an alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
may form an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
. Nonetheless, the total number of π bond must decrease in the process. The π carbon–carbon bond is also necessary for this process.
The reaction equation of hydrogenation of ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene is ...
to form ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petr ...
is:
*H2C=CH2 + H2→H3C−CH3
The hydrogenation reaction usually requires catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s to increase its rate.
The total number of hydrogen that can be added to an unsaturated hydrocarbon depends on its degree of unsaturation
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of r ...
. An unsaturated hydrocarbon with formula of CXHY can have 2X+2−Y hydrogen atoms at most added to it. This will make the molecule become saturated.
Halogenation
Similar as hydrogen, the heterolysis ofhalogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
(X2) will produce an electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
X+ ion, after which it will be attacked by the electron on the π bond. Different from hydrogen, halogenation will produce halonium ion
A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure where X is any halogen and no restrictions on R, this structure can be cyclic or an open chain molecular structure. Halo ...
s as intermediate instead of carbocations in most other cases. The halonium cation leaves limited space for the X− ion to attack and will only turn into a trans
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of".
Used alone, trans may refer to:
Arts, entertainment, and media
* Trans (festival), a former festival in Belfast, Northern Ireland, United Kingdom
* ''Trans'' (film ...
product. The net result of halogenation is a decrease of one π bond and an increase two carbon-halogen σ bonds on the 2 carbons.
 The reaction equation for bromine addition of
The reaction equation for bromine addition of ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene is ...
, for example, is:
*H2C=CH2 + Br2→H2CBr−CH2Br (trans)
Bromine test In organic chemistry, the bromine test is a qualitative test for the presence of unsaturation (carbon-to-carbon double or triple bonds), phenols and anilines.
An unknown sample is treated with a small amount of elemental bromine in an organic sol ...
is used to test the saturation of hydrocarbons. The test involves the addition of bromine water to the unknown hydrocarbon; If the bromine water
Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (Br2) dissolved in water (H2O). It is often used as a reactive in chemical assays of recognition for substances which react with bromine in an aqueous environment wi ...
is decolourized by the hydrocarbon, which is due to halogenation reaction, it can then be concluded that the hydrocarbon is unsaturated. If it is not decolourized, then it is saturated.
The bromine test can also be used as an indication of the degree of unsaturation
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of r ...
for unsaturated hydrocarbons. Bromine number
In chemistry, the bromine number is the amount of bromine () in grams absorbed by of a sample. The number indicates the degree of unsaturation.
The bromine number is useful as a measure of aliphatic unsaturation in gasoline samples. The Califo ...
is defined as gram of bromine able to react with 100g of product. Similar as hydrogenation, the halogenation of bromine is also depend on the number of π bond. A higher bromine number indicates higher degree of unsaturation.
Hydration
The π bond of unsaturated hydrocarbons are also ready to accept H+ and OH− from water. The reaction usually involvesstrong acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions. ...
as catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. That is because the first step of mechanism of hydration involves the π bond deprotonate a H+ from the strong acid to form a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
. The net result of the reaction will be an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
.
The reaction equation for hydration of ethene is:
*H2C=CH2 + H2O→
The π bonds in triple bond are also able to go under hydration in acidic condition and form enols. However, the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
will not be a product but an intermediate, and the final product will be a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
. The enol intermediate goes under tautomerization
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
and form the more stable ketone.
The reaction equation of hydration of ethyne to form acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
is:
*HC≡CH + H2O → H2C=CH−OH
*H2C=CH−OH ⇌ H3C−CHO
Hydrohalogenation
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a p ...
. The hydrohalogenation of alkene will result in haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
, and hydrohalogenation of alkyne will result in vinyl halide
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl ...
. The hydrohalogenation of alkyne is much slower than the alkene.
The reaction equation of HBr addition to ethene is:
*H2C=CH2 + HBr→
Oxidation
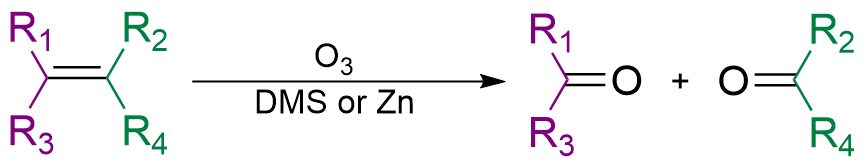
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of unsaturated hydrocarbons depends on the strength of oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In ot ...
. A weak oxidizing agent will lead to dihydroxylation
Dihydroxylation is the process by which an alkene is converted into a vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal (typically osmium or ...
, removal of one π bond to form two σ bonds with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
. Dihydroxylation of alkene produces diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
, and dihydroxylation of alkyne produces vicinal dicarbonyl.
A stronger oxidizing agent, for example KMnO4 or ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
, will lead to oxidative cleavage
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carr ...
. In this case, the π bond breakes with the σ bond, dividing the hydrocarbon molecule into two. Oxygen bonds with the remaining two π bonds separately. Oxidative cleavage of alkene produces ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s or aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s, depending on the place of double bond, and cleavage of alkynes produces carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
.
Allylic substitution
The π bond in unsaturated hydrocarbons will lower the dissociation energy of the allylic C−H bonds, which are C−H bonds of the carbon that is adjacent to the sp2 carbons. As a result, the free radical substitution reaction will be favored over the addition reactions. An example of this is NBS bromination reaction with alkene. The N−Br bond in NBS is weak so that much Br free radical will form. The free radical will attack the weakened allylic hydrogens and substitute them with bromine atoms. The reaction equation is: *RCH2CH=CH2 + (CH2CO)2NBr → RCHBrCH=CH2 + RCH=CHCH2Br + (CH2CO)2N The reaction will produce two isomers with bromine attached to different carbons. The reaction requires high amount of Br free radicals instead of electrophilic Br+ ions, which will go under addition reaction. NBS is essential to make such condition. If hydrocarbon groups are attached to allylic carbon, it will make this carbon be more saturated. According toZaitsev's Rule
In organic chemistry, Zaitsev's rule (or Saytzeff's rule, Saytzev's rule) is an empirical rule for predicting the favored alkene product(s) in elimination reactions. While at the University of Kazan, Russian chemist Alexander Zaitsev studied a vari ...
, this carbon will form a more stable carbocation intermediate. As a result, allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.
In reaction conditions that favor a SN1 react ...
will occur, and the π bond will move to this carbon. This will produce a major product of bromine substituted to the carbon four bonds away from the hydrocarbon group.
Cycloaddition
conjugated diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclatur ...
and a substituted alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, Diels-Alder reaction will occur that forms a cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light an ...
. Such reaction is highly selective in stereochemistry.
Alkynes, under metal catalysts, for example cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
, can also go under cycloaddition reaction called alkyne trimerization
In organic chemistry, an alkyne trimerisation is a +2+2nbsp;cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicab ...
. Three alkynes goes under a "2+2+2" cyclization reaction and rapidly join to form a benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
. Trimerization of different alkenes are usually not selective, but specially designed catalysts may increase the selectivity.
React as ligand
 The delocalized π bond in unsaturated hydrocarbons provide high electron density, making the molecule possible to become a metal
The delocalized π bond in unsaturated hydrocarbons provide high electron density, making the molecule possible to become a metal ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
. In alkene ligand, the bonding structure can be described by Dewar–Chatt–Duncanson model
The Dewar–Chatt–Duncanson model is a model in organometallic chemistry that explains the chemical bonding in transition metal alkene complexes. The model is named after Michael J. S. Dewar, Joseph Chatt and L. A. Duncanson.
The alkene donat ...
. In this case, the π electron density are donated to the metal d orbitals. The stronger the donation is, the stronger the back bonding
In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a ''π-acceptor ligand''. It is especially common in the organometallic chemi ...
from the metal d orbital to π* anti-bonding orbital of the alkene. This effect reduces the bond order of the alkene and increases the C-C bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
. As a result, the metal forms a small ring structure with the two carbons.
The DCD model can also describe the alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
ligand structure. Metal complex can also be intermediate of trimerization
In chemistry, a trimer (; ) is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors ofte ...
of alkynes, so metals can be catalysts of the reaction.
The synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
of alkene ligand complexes can be described as an electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Che ...
reaction.
Similar as linear unsaturated hydrocarbons, the arene also have delocalized π bonds able to donate to metals to form complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
. In cases like benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
, the carbons donate equally electron density to the metal, whereas in some other cases, carbons donate differently to the metal, causing the arene to bent or dearomatize.
Application
Unsaturated hydrocarbons are widely used aspesticide
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampri ...
s, fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work. The concept was originally applied solely to those materials capable of releasing chemical energy but ...
, paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s, and many other necessities. Below is a table of some common commercial unsaturated hydrocarbons.
Unsaturated hydrocarbons are also used in many chemical reactions to synthesize other compounds. One of their utility in this area is to be used as monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
s in polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
reactions. In these reactions, simple monomer unit molecules react and bind with each other either linearly or nonlinearly to synthesize macromolecules, yielding either polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
chains or 3D structures. During polymerization, the double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
in the monomers usually turns into a single bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of th ...
so that two other monomer molecules can attach on both sides. Some products of polymerization reactions are closely related to our daily life. For example, one of the common types of plastic, polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bo ...
, is the polymerization product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
. Also, Styrofoam
Styrofoam is a trademarked brand of closed-cell extruded polystyrene foam (XPS), commonly called "Blue Board", manufactured as foam continuous building insulation board used in walls, roofs, and foundations as thermal insulation and water barrie ...
(polystyrene) is the synthesized from the polymerization of styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
.
See also
*Saturated hydrocarbon
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
References
{{DEFAULTSORT:Unsaturated Hydrocarbon Hydrocarbons