Tsuji–Trost Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a  This work was first pioneered by Jirō Tsuji in 1965 and, later, adapted by
This work was first pioneered by Jirō Tsuji in 1965 and, later, adapted by
 The scope of the reaction was expanded only gradually until Trost discovered the next big breakthrough in 1973. While attempting to synthesize acyclic sesquiterpene homologs, Trost ran into problems with the initial procedure and was not able to
The scope of the reaction was expanded only gradually until Trost discovered the next big breakthrough in 1973. While attempting to synthesize acyclic sesquiterpene homologs, Trost ran into problems with the initial procedure and was not able to  These conditions were then tested out for other substrates and some led to "essentially instantaneous reaction at room temperature." Soon after, he developed a way to use these ligands for asymmetric synthesis. Not surprisingly, this spurred on many other investigations of this reaction and has led to the important role that this reaction now holds in synthetic chemistry.
These conditions were then tested out for other substrates and some led to "essentially instantaneous reaction at room temperature." Soon after, he developed a way to use these ligands for asymmetric synthesis. Not surprisingly, this spurred on many other investigations of this reaction and has led to the important role that this reaction now holds in synthetic chemistry.
 First, the palladium coordinates to the alkene, forming a η2 -allyl- Pd0
First, the palladium coordinates to the alkene, forming a η2 -allyl- Pd0
 This trend is explained by examining the mechanisms of nucleophilic attack. "Soft" nucleophiles attack the carbon of the allyl group, while "hard" nucleophiles attack the metal center, followed by reductive elimination.
This trend is explained by examining the mechanisms of nucleophilic attack. "Soft" nucleophiles attack the carbon of the allyl group, while "hard" nucleophiles attack the metal center, followed by reductive elimination.

 Another Tsuji–Trost reaction was used during the initial stages of the synthesis of (−)- neothiobinupharidine. This recent work demonstrates the ability of this reaction to give highly diastereoselective (10:1) and enantioselective (97.5:2.5) products from
Another Tsuji–Trost reaction was used during the initial stages of the synthesis of (−)- neothiobinupharidine. This recent work demonstrates the ability of this reaction to give highly diastereoselective (10:1) and enantioselective (97.5:2.5) products from 
 This simple, high-throughput method to detect palladium by monitoring fluorescence has been shown to be useful in monitoring palladium levels in metal ores,
This simple, high-throughput method to detect palladium by monitoring fluorescence has been shown to be useful in monitoring palladium levels in metal ores,
Org. Synth. 1989, 67, 105
Org. Synth. 2009, 86, 47
* example of tsuji-trost reaction in total synthesis see : http://www.biocis.u-psud.fr/IMG/pdf/concise_total_synthesis_of_Minfiensine.pdf the second reaction found on website of the biocis team : http://www.biocis.u-psud.fr/spip.php?article332 {{DEFAULTSORT:Tsuji-Trost reaction Organic reactions Substitution reactions Palladium Name reactions Allyl complexes
palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
- catalysed substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
involving a substrate that contains a leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
in an allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
position. The palladium catalyst first coordinates with the allyl group and then undergoes oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
, forming the -allyl complex. This allyl complex
Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usual ...
can then be attacked by a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, resulting in the substituted product.
Barry Trost
Barry M. Trost (born June 13, 1941, in Philadelphia) is an American chemist who is the Job and Gertrud Tamaki Professor Emeritus in the School of Humanities and Sciences at Stanford University. The Tsuji–Trost reaction and the Trost ligand ar ...
in 1973 with the introduction of phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands.
The scope of this reaction has been expanded to many different carbon, nitrogen, and oxygen-based nucleophiles, many different leaving groups, many different phosphorus, nitrogen, and sulfur-based ligands, and many different metals (although palladium is still preferred).
The introduction of phosphine ligands led to improved reactivity and numerous asymmetric allylic alkylation strategies. Many of these strategies are driven by the advent of chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
, which are often able to provide high enantioselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
and high diastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have d ...
under mild conditions. This modification greatly expands the utility of this reaction for many different synthetic applications. The ability to form carbon-carbon, carbon-nitrogen, and carbon-oxygen bonds under these conditions, makes this reaction very appealing to the fields of both medicinal chemistry and natural product synthesis.
History
In 1962, Smidt published work on the palladium-catalysed oxidation ofalkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
to carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
groups. In this work, it was determined that the palladium catalyst activated the alkene for the nucleophilic attack of hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
. Gaining insight from this work, Tsuji hypothesized that a similar activation could take place to form carbon-carbon bonds.
In 1965, Tsuji reported work that confirmed his hypothesis. By reacting an allylpalladium chloride dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
** TH-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* ...
with the sodium salt of diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds ...
, the group was able to form a mixture of monoalkylated and dialkylated product.
 The scope of the reaction was expanded only gradually until Trost discovered the next big breakthrough in 1973. While attempting to synthesize acyclic sesquiterpene homologs, Trost ran into problems with the initial procedure and was not able to
The scope of the reaction was expanded only gradually until Trost discovered the next big breakthrough in 1973. While attempting to synthesize acyclic sesquiterpene homologs, Trost ran into problems with the initial procedure and was not able to alkylate
An alkylation unit (alky) is one of the conversion processes used in petroleum refineries. It is used to convert isobutane and low-molecular-weight alkenes (primarily a mixture of propene and butene) into alkylate, a high octane gasoline componen ...
his substrates. These problems were overcome with the addition of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
to the reaction mixture.
 These conditions were then tested out for other substrates and some led to "essentially instantaneous reaction at room temperature." Soon after, he developed a way to use these ligands for asymmetric synthesis. Not surprisingly, this spurred on many other investigations of this reaction and has led to the important role that this reaction now holds in synthetic chemistry.
These conditions were then tested out for other substrates and some led to "essentially instantaneous reaction at room temperature." Soon after, he developed a way to use these ligands for asymmetric synthesis. Not surprisingly, this spurred on many other investigations of this reaction and has led to the important role that this reaction now holds in synthetic chemistry.
Mechanism
Starting with a zerovalent palladium species and a substrate containing a leaving group in the allylic position, the Tsuji–Trost reaction proceeds through thecatalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
outlined below.
 First, the palladium coordinates to the alkene, forming a η2 -allyl- Pd0
First, the palladium coordinates to the alkene, forming a η2 -allyl- Pd0 Π complex
Pi () is a mathematical constant equal to a circle's circumference divided by its diameter.
Pi, π or Π may also refer to:
Language and typography
* Pi (letter), the 17th letter of the Greek alphabet
* Pi characters, uncommon characters in t ...
. The next step is oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
in which the leaving group is expelled with inversion of configuration and a η3 -allyl- PdII is created (also called ionization). The nucleophile then adds to the allyl group regenerating the η2 -allyl-Pd0 complex. At the completion of the reaction, the palladium detaches from the alkene and can start again in the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
.
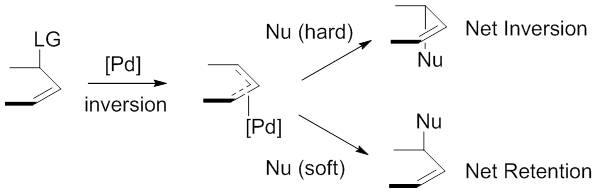
"Hard" versus "soft" nucleophiles
The nucleophiles used are typically generated from precursors (pronucleophiles)in situ
is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is use ...
after their deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
with base. These nucleophiles are then subdivided into "hard" and "soft" nucleophiles using a paradigm for describing nucleophiles that largely rests on the of their conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the rever ...
s. "Hard" nucleophiles typically have conjugate acids with greater than 25, while "soft" nucleophiles typically have conjugate acids with less than 25.
This descriptor is important because of the impact these nucleophiles have on the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
of the product. Stabilized or "soft" nucleophiles invert the stereochemistry of the -allyl complex. This inversion in conjunction with the inversion in stereochemistry associated with the oxidative addition of palladium yields a net retention of stereochemistry. Unstabilized or "hard" nucleophiles, on the other hand, retain the stereochemistry of the -allyl complex, resulting in a net inversion of stereochemistry.
 This trend is explained by examining the mechanisms of nucleophilic attack. "Soft" nucleophiles attack the carbon of the allyl group, while "hard" nucleophiles attack the metal center, followed by reductive elimination.
This trend is explained by examining the mechanisms of nucleophilic attack. "Soft" nucleophiles attack the carbon of the allyl group, while "hard" nucleophiles attack the metal center, followed by reductive elimination.
Phosphine ligands
Phosphine ligands, such as triphenylphosphine or theTrost ligand
The Trost ligand is a diphosphine used in the palladium- catalyzed Trost asymmetric allylic alkylation. Other C2-symmetric ligands derived from ''trans''-1,2-diaminocyclohexane (DACH) have been developed, such as the (''R'',''R'')-DACH- naphthy ...
, have been used to greatly expand the scope of the Tsuji–Trost reaction. These ligands can modulate the properties of the palladium catalyst such as steric bulk
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
as well as the electronic properties. Importantly, these ligands can also instill chirality to the final product, making it possible for these reactions to be carried out asymmetrically as shown below.
Allylic asymmetric substitution
The enantioselective version of the Tsuji–Trost reaction is called the Trost asymmetric allylic alkylation (Trost AAA) or simply, asymmetric allylic alkylation (AAA). These reactions are often used in asymmetric synthesis. The reaction was originally developed with a palladium catalyst supported by theTrost ligand
The Trost ligand is a diphosphine used in the palladium- catalyzed Trost asymmetric allylic alkylation. Other C2-symmetric ligands derived from ''trans''-1,2-diaminocyclohexane (DACH) have been developed, such as the (''R'',''R'')-DACH- naphthy ...
, although suitable conditions have greatly expanded since then.
Enantioselectivity can be imparted to the reaction during any of the steps aside from the decomplexation
In chemistry, decomplexation refers to the removal of a ligand from a coordination complex. Decomplexation is of particular interest when the ligand has been synthesized within the coordination sphere of the metal, as is often the case in organome ...
of the palladium from the alkene since the stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
is already set at that point. Five main ways have been conceptualized to take advantage of these steps and yield enantioselective reaction conditions.
These methods of enantiodiscrimination were previously reviewed by Trost:
# Preferential ionization via enantioselective olefin complexation
# Enantiotopic ionization of leaving groups
# Attack at enantiotopic ends of the allyl complex
# Enantioface exchange in the -allyl complex
# Differentiation of prochiral nucleophile faces
The favored method for enantiodiscrimination is largely dependent on the substrate of interest, and in some cases, the enantioselectivity may be influenced by several of these factors.
Scope
Nucleophiles
Many different nucleophiles have been reported to be effective for this reaction. Some of the most common nucleophiles include malonates, enolates, primary alkoxides,carboxylates
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... ...
, phenoxides
Phenolates (also called phenoxides) are anions, salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenolate hydrolyze in aq ...
, amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
, azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
, sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this gro ...
s, imides
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications ...
, and sulfones
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
.
Leaving groups
The scope of leaving groups has also been expanded to include a number of different leaving groups, althoughcarbonates
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . ...
, phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
, phosphates
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphor ...
, halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluo ...
and carboxylates
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... ...
are the most widely used.
"Hard" and "soft" nucleophiles
Recent work has demonstrated that the scope of "soft" nucleophiles can be expanded to include some pronucleophiles that have much higher than ~ 25. Some of these "soft" nucleophiles have ranging all the way to 32, and even more basic pronucleophiles (~44) have been shown to act as soft nucleophiles with the addition ofLewis acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
that help to facilitate deprotonation. The improved pKa range of "soft" nucleophiles is critical because these nucleophiles are the only ones that have been explored for enantioselective reactions until very recently (although non-enantioselective reactions of "hard" nucleophiles have been known for some time). By increasing the scope of pronucleophiles that act as "soft" nucleophiles, these substrates can also be incorporated into enantioselective reactions using previously reported and well characterized methods.

Ligands
Building on the reactivity of the triphenylphosphine ligand, the structure of ligands used for the Tsuji–Trost reaction quickly became more complex. Today, these ligands may contain phosphorus, sulfur, nitrogen or some combination of these elements, but most studies have concentrated on the mono- and diphosphine ligands. These ligands can be further classified based on the nature of their chirality, with some ligands containing central chirality on the phosphorus or carbon atoms, some containing biarylaxial chirality
In chemistry, axial chirality is a special case of chirality (chemistry), chirality in which a molecule contains two pairs of chemical groups in a non-planar arrangement about an axis of chirality so that the molecule is not superposable on its mi ...
, and others containing planar chirality Planar chirality, also known as 2D chirality, is the special case of chirality for two dimensions.
Most fundamentally, planar chirality is a mathematical term, finding use in chemistry, physics and related physical sciences, for example, in astrono ...
.
Diphosphine ligands with central chirality emerged as an effective type of ligand (particularly for asymmetric allylic alkylation procedures) with the Trost Ligand being one such example.
Phosphinooxazolines (PHOX) ligands have been employed in the AAA, particularly with carbon-based nucleophiles.
Additional substrates
The reaction substrate has also been extended toallene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
s. In this specific ring expansion the AAA reaction is also accompanied by a Wagner–Meerwein rearrangement
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement rearrangement reaction, reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.
They can be described as cationic ,2sig ...
:
Applications
Pharmaceutical/natural products synthesis
The ability to form carbon-carbon, carbon-nitrogen, and carbon-oxygen bonds enantioselectively under mild conditions makes the Trost asymmetric allylic alkylation extremely appealing for the synthesis of complex molecules. An example of this reaction is the synthesis of an intermediate in the combined total synthesis of galantamine andmorphine
Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies (''Papaver somniferum''). It is mainly used as an analgesic (pain medication). There are ...
Trost, B. M.; Tang, W.; Toste, F. D. "Divergent Enantioselective Synthesis of (−)-Galantamine and (−)-Morphine." ''J. Am. Chem. Soc.'' 2005, ''127'', 14785–14803. . with 1 mol% i-allylpalladium chloride dimer 3 mol% (''S,S'') Trost ligand
The Trost ligand is a diphosphine used in the palladium- catalyzed Trost asymmetric allylic alkylation. Other C2-symmetric ligands derived from ''trans''-1,2-diaminocyclohexane (DACH) have been developed, such as the (''R'',''R'')-DACH- naphthy ...
, and triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
in dichloromethane
Dichloromethane (DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with ...
at room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
. These conditions result in the formation of the (−)-enantiomer of the aryl ether in 72% chemical yield
In chemistry, yield, also known as reaction yield or chemical yield, refers to the amount of product obtained in a chemical reaction. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis ...
and 88% enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a sing ...
.
achiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
starting material with only a small amount of catalyst ( 1%).

Palladium detection
Aside from the practical application of this reaction in medicinal chemistry and natural product synthesis, recent work has also used the Tsuji–Trost reaction to detect palladium in various systems. This detection system is based on a non-fluorescent
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with color ...
fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to Triarylmethane dye, triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. ...
-derived sensor (longer-wavelength sensors have also recently been developed for other applications) that becomes fluorescent only in the presence of palladium or platinum.
This palladium/platinum sensing ability is driven by the Tsuji–Trost reaction. The sensor contains an allyl group with the fluorescein functioning as the leaving group. The -allyl complex is formed and after a nucleophile attacks, the fluorescein is released, yielding a dramatic increase in fluorescence.
 This simple, high-throughput method to detect palladium by monitoring fluorescence has been shown to be useful in monitoring palladium levels in metal ores,
This simple, high-throughput method to detect palladium by monitoring fluorescence has been shown to be useful in monitoring palladium levels in metal ores, pharmaceutical products
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the m ...
, and even in living cells
The cell is the basic structural and functional unit of all life, forms of life. Every cell consists of cytoplasm enclosed within a Cell membrane, membrane; many cells contain organelles, each with a specific function. The term comes from the ...
. With the ever-increasing popularity of palladium catalysis, this type of quick detection should be very useful in reducing the contamination of pharmaceutical products and preventing the pollution of the environment with palladium and platinum.
References
External links
Org. Synth. 1989, 67, 105
Org. Synth. 2009, 86, 47
* example of tsuji-trost reaction in total synthesis see : http://www.biocis.u-psud.fr/IMG/pdf/concise_total_synthesis_of_Minfiensine.pdf the second reaction found on website of the biocis team : http://www.biocis.u-psud.fr/spip.php?article332 {{DEFAULTSORT:Tsuji-Trost reaction Organic reactions Substitution reactions Palladium Name reactions Allyl complexes