|
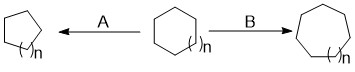
Ring Expansion Reaction
Ring expansion and ring contraction reactions in the course of organic synthesis refer to a set of reactions which can lead to the expansion or contraction of an existing ring. This often makes it possible to access structures that would be difficult if not impossible to synthesise with single cyclization reactions. Ring expansions are valuable because they allow access to larger systems that are difficult to synthesize through a single cyclization due to the slow rate of formation. Ring contractions are useful for making smaller, more strained rings from larger rings. Expansions are classified by the mechanism of expansion and the atom(s) added; contractions are characterized simply by the reactive intermediate which performs the contraction. Description In the course of an organic synthesis, a chemist often needs to form a new or alter an existing ring. Ring expansion and ring contraction reactions are used to expand or contract an existing ring, often making it possible t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
General Contraction Expansion
A general officer is an officer of high rank in the armies, and in some nations' air forces, space forces, and marines or naval infantry. In some usages the term "general officer" refers to a rank above colonel."general, adj. and n.". OED Online. March 2021. Oxford University Press. https://www.oed.com/view/Entry/77489?rskey=dCKrg4&result=1 (accessed May 11, 2021) The term ''general'' is used in two ways: as the generic title for all grades of general officer and as a specific rank. It originates in the 16th century, as a shortening of ''captain general'', which rank was taken from Middle French ''capitaine général''. The adjective ''general'' had been affixed to officer designations since the late medieval period to indicate relative superiority or an extended jurisdiction. Today, the title of ''general'' is known in some countries as a four-star rank. However, different countries use different systems of stars or other insignia for senior ranks. It has a NATO rank scal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generalized Wolf Rearrangement Mechanism
A generalization is a form of abstraction whereby common properties of specific instances are formulated as general concepts or claims. Generalizations posit the existence of a domain or set of elements, as well as one or more common characteristics shared by those elements (thus creating a conceptual model). As such, they are the essential basis of all valid deductive inferences (particularly in logic, mathematics and science), where the process of verification is necessary to determine whether a generalization holds true for any given situation. Generalization can also be used to refer to the process of identifying the parts of a whole, as belonging to the whole. The parts, which might be unrelated when left on their own, may be brought together as a group, hence belonging to the whole by establishing a common relation between them. However, the parts cannot be generalized into a whole—until a common relation is established among ''all'' parts. This does not mean that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinacol Rearrangment To A 5,7 Ring System
Pinacol is a white solid organic compound. It is a diol that has hydroxyl groups (-OH) on vicinal carbon atoms. Preparation It may be produced by the pinacol coupling reaction from acetone: Reactions As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g. by heating with sulfuric acid: Pinacol can be used with borane and boron trichloride to produce useful synthetic intermediates such as pinacolborane Pinacolborane is the borane with the formula (CH3)4C2O2BH. Often pinacolborane is abbreviated HBpin. It features a boron hydride functional group incorporated in a five-membered C2O2B ring. Like related boron alkoxides, pinacolborane is monomeric ..., bis(pinacolato)diboron,{{OrgSynth , collvol = 10 , collvolpages = 115 , year = 2004 , prep = v77p0176 , title = Bis(pinacolato)diboron , author1 = Tatsuo Ishiyama, author2= Miki Murata, author3=Taka-aki Ahiko, author4-link= Norio Miyaura, author4=Norio Miyaura and pinacolchloroborane. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generalized Favorskii Rearrangement Mechansim
A generalization is a form of abstraction whereby common properties of specific instances are formulated as general concepts or claims. Generalizations posit the existence of a domain or set of elements, as well as one or more common characteristics shared by those elements (thus creating a conceptual model). As such, they are the essential basis of all valid deductive inferences (particularly in logic, mathematics and science), where the process of verification is necessary to determine whether a generalization holds true for any given situation. Generalization can also be used to refer to the process of identifying the parts of a whole, as belonging to the whole. The parts, which might be unrelated when left on their own, may be brought together as a group, hence belonging to the whole by establishing a common relation between them. However, the parts cannot be generalized into a whole—until a common relation is established among ''all'' parts. This does not mean that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Favorskii Rearrangement
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds. History The reaction is named for the Russian chemist Alexei Yevgrafovich Favorskii Reaction mechanism The reaction mechanism is thought to involve the formation of an enolate on the side of the ketone away from the chlorine atom. This enolate cyclizes to a cyclopropanone intermediate which is then attacked by the hydroxide nucleophile. The second step has also been proposed to be stepwise process, with chloride anion lea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RIng Contraction Mechanisms
Ring may refer to: * Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry * To make a sound with a bell, and the sound made by a bell :(hence) to initiate a telephone connection Arts, entertainment and media Film and literature * ''The Ring'' (franchise), a Japanese horror media franchise based on the novel series by Koji Suzuki ** ''Ring'' (novel series) *** ''Ring'' (Suzuki novel), 1991 ** ''Ring'' (film), or ''The Ring'', a 1998 Japanese horror film by Hideo Nakata *** ''The Ring'' (2002 film), an American horror film, remake of the 1998 Japanese film ** ''Ring'' (1995 film), a TV film ** ''Rings'' (2005 film), a short film by Jonathan Liebesman ** ''Rings'' (2017 film), an American horror film * ''Ring'' (Baxter novel), a 1994 science fiction novel * ''Ring'' (Alexis novel), a 2021 Canadian novel by André Alexis Gaming * ''Ring'' (video game), 1998 * Rings (''Sonic the Hedgehog''), a collectible in ''Sonic the Hedgehog'' games Music ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beckmann Rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams. The Beckmann rearrangement is often catalyzed by acid; however, other reagents have been known to promote the rearrangement. These include tosyl chloride, thionyl chloride, phosphorus pentachloride, phosphorus pentoxide, triethylamine, sodium hydroxide, trimethylsilyl iodide among others. The Beckmann fragmentation is another reaction that often competes with the rearrangement, though careful selection of promoting reagent and solvent conditions can favor the formation of one over the other, sometimes giving almost exclusively one product. The rearrangement occurs stereospecifically for ketoximes and N-chloro/N-fluoro imines, with the migrating group being anti-periplanar to the leaving gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Reaction
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buchner Ring Expansion
The Buchner ring expansion is a two-step organic C-C bond forming reaction used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring. History The Buchner ring expansion reaction was first used in 1885 by E. Buchner and T. Curtius who prepared a carbene from ethyl diazoacetate for addition to benzene using both thermal and photochemical pathways in the synthesis of cycloheptatriene derivatives. The resulting product was a mixture of four isomeric carboxylic acids. Variations in the reaction arise from methods of carbene preparation. Advances in organometallic chemistry have resulted in increased selectivity of cycloheptatriene derivatives. In the 1980s it was found that dirhodium catalysts provide single cyclopropane isomers in high yields. Applications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |