trimethylolpropane phosphite on:
[Wikipedia]
[Google]
[Amazon]
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a


phosphite ester
The general structure of a phosphite ester showing the lone pairs on the P
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of a ...
used as a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
in organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
. Trimethylolpropane phosphite is sometimes abbreviated to EtCage. It is a white solid that is soluble in organic solvents. It is also highly toxic.
Preparation and reactions
It is prepared by reaction oftrimethylolpropane 300 C-->
Trimethylolpropane (TMP) is the organic compound with the formula CH3CH2C(CH2OH)3. This colourless to white solid with a faint odor is a triol. Containing three hydroxy functional groups, TMP is a widely used building block in the polymer ...
with phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic a ...
or by transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can ...
with trimethylphosphite
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic che ...
:
:P(OMe)3 + EtC(CH2OH)3 → 3 MeOH + EtC(CH2O)3P
The first member of this series was derived from trimethylolethane
Trimethylolethane (TME) is the organic compound with the formula CH3C(CH2OH)3. This colorless solid is a triol, as it contains three hydroxy functional groups. More specifically, it features three primary alcohol groups in a compact neopentyl stru ...
, but these derivatives are often poorly soluble. For this reason, the ethyl derivative has received more attention.
Reactions
The compound forms an isolable ozonide, which degrades above 0 °C to release singlet O2.Coordination chemistry
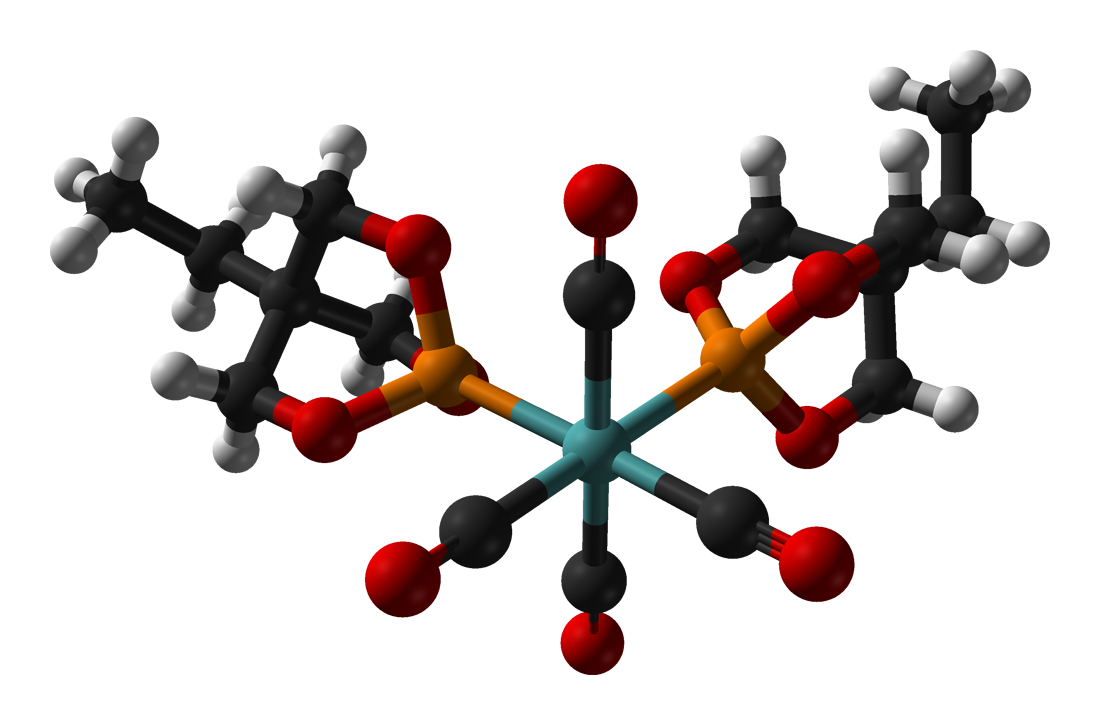
Several EtCage complexes are known, since the ligand is highly basic (for a phosphite) and has a smallligand cone angle
In coordination chemistry, the ligand cone angle (a common example being the Tolman cone angle or ''θ'') is a measure of the steric bulk of a ligand in a transition metal coordination complex. It is defined as the solid angle formed with the me ...
(101°). Illustrative complexes include EtCage)2Mo(CO)4 r4(CO)11(EtCage)and (CpMe5)RuCl(EtCage)2, shown below.



Safety
Trimethylolpropane phosphite is very toxic and is aconvulsant A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent exc ...
. LD50 is 1.1 mg per kg bodyweight (mice, i.p.).
References