Transmembrane Alpha-helix on:
[Wikipedia]
[Google]
[Amazon]
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an
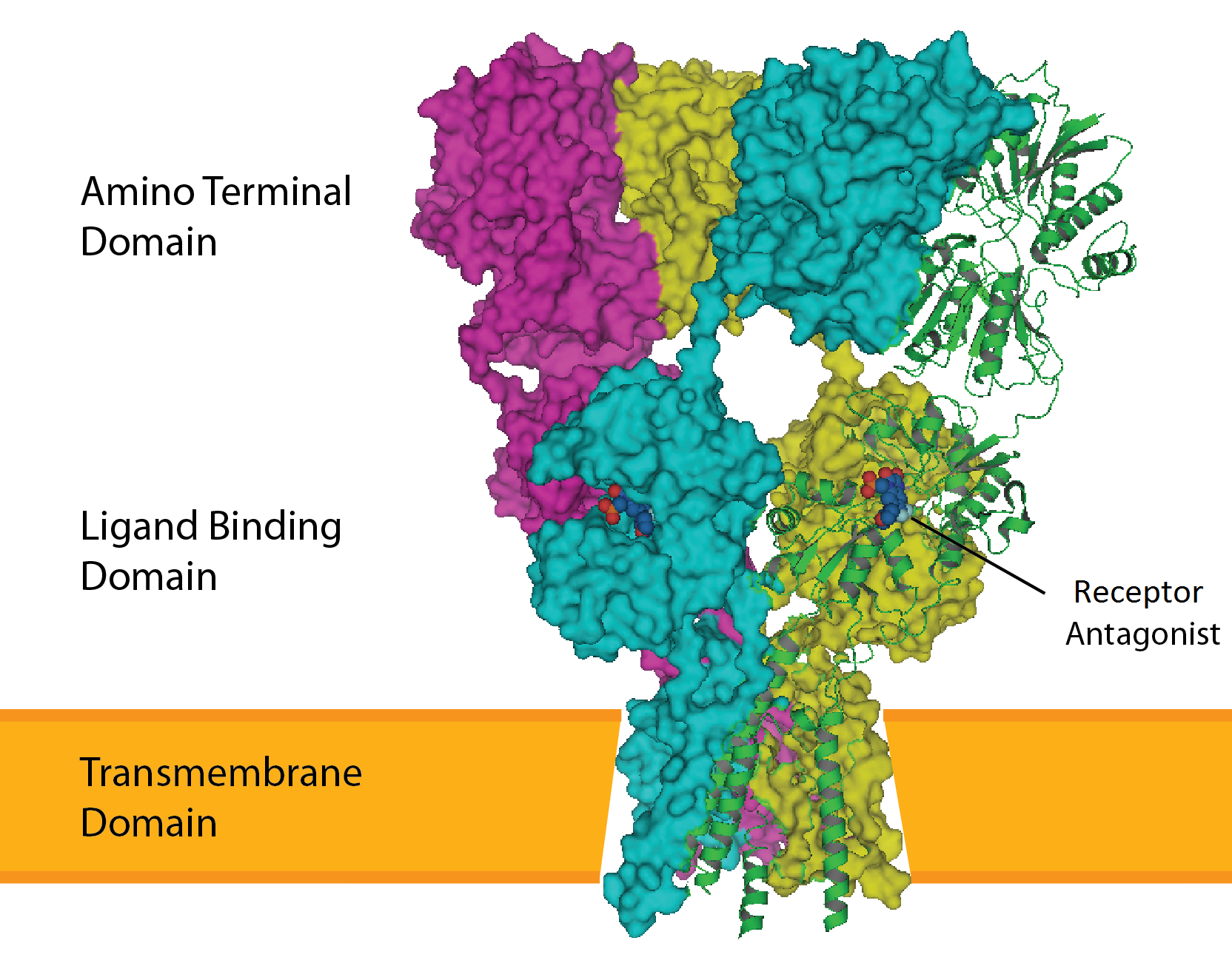 *Facilitating molecular transport of molecules such as ions and proteins across
*Facilitating molecular transport of molecules such as ions and proteins across
TMHMM
alpha helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues e ...
topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of io ...
can contain polar residues. TMDs vary greatly in length, sequence, and hydrophobicity, adopting organelle-specific properties.
Functions of transmembrane domains
Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across
*Facilitating molecular transport of molecules such as ions and proteins across biological membrane
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell from the external environment or creates intracellular compartments by serving as a boundary between one part of the ce ...
s; usually hydrophilic residues and binding sites in the TMDs help in this process.
*Signal transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellula ...
across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellular signals. TMDs then propagate those signals across the membrane to induce an intracellular effect.
* Assisting in vesicle fusion; the function of TMDs is not well understood, but they have been shown to be critical for the fusion reaction, possibly as a result of TMDs affecting the tension of the lipid bilayer.
* Mediating transport and sorting of transmembrane proteins; TMDs have been shown to work in tandem with cytosolic sorting signals, with length and hydrophobicity being the main determinants in TDM sorting. Longer and more hydrophobic TMDs aid in sorting proteins to the cell membrane, whereas shorter and less hydrophobic TMDs are used to retain proteins in the endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
and the Golgi apparatus. The exact mechanism of this process is still unknown.
Identification of transmembrane helices
Transmembrane helices are visible in structures of membrane proteins determined byX-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. They may also be predicted on the basis of hydrophobicity scales. Because the interior of the bilayer
A bilayer is a double layer of closely packed atoms or molecules.
The properties of bilayers are often studied in condensed matter physics, particularly in the context of semiconductor devices, where two distinct materials are united to form junc ...
and the interiors of most proteins of known structure are hydrophobic, it is presumed to be a requirement of the amino acids that span a membrane that they be hydrophobic as well. However, membrane pumps and ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of io ...
s also contain numerous charged and polar residues within the generally non-polar transmembrane segments.
Using "hydrophobicity analysis" to predict transmembrane helices enables a prediction in turn of the "transmembrane topology" of a protein; i.e. prediction of what parts of it protrude into the cell, what parts protrude out, and how many times the protein chain crosses the membrane.
Transmembrane helices can also be identified ''in silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for 'in silicon' (correct la, in silicio), referring to silicon in computer chips. It ...
'' using the bioinformatic toolTMHMM
The role of membrane protein biogenesis and quality control factors
Since protein translation occurs in the cytosol (an aqueous environment), factors that recognize the TMD and protect them in this hostile environment are required. Additional factors that allow the TMD to be incorporated into the target membrane (i.e.endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
or other organelles) are also required. Factors also detect TMD misfolding within the membrane and perform quality control functions. These factors must be able to recognize a highly variable set of TMDs and can be segregated into those active in the cytosol or active in the membrane.
Cytosolic Recognition Factors
Cytosolic recognition factors are thought to use two distinct strategies. In the co-translational strategy the recognition and shielding are coupled to protein synthesis. Genome wide association studies indicate the majority of membrane proteins targeting the endoplasmic reticulum are handled by the signal recognition particle which is bound to the ribosomal exit tunnel and initiates recognition and shielding as protein is translated. The second strategy involves tail-anchored proteins, defined by a single TMD located close to the carboxyl terminus of the membrane protein. Once translation is completed, the tail-anchored TMD remains in the ribosomal exit tunnel, and an ATPase mediates targeting to the endoplasmic reticulum. Examples of shuttling factors include TRC40 in higher eukaryotes and Get3 in yeast. Furthermore, general TMD-binding factors protect against aggregation and other disrupting interactions. SGTA andcalmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the bind ...
are two well-known general TMD-binding factors. Quality control of membrane proteins involve TMD-binding factors that are linked to ubiquitination proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
Proteasomes are part of a major mechanism by w ...
system.
Membrane Recognition Factors
Once transported, factors assist with insertion of the TMD across the hydrophilic layer phosphate "head" group of thephospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
membrane. Quality control factors must be able to discern function and topology, as well as facilitate extraction to the cytosol. The signal recognition particle transports membrane proteins to the Sec translocation channel, positioning the ribosome exit tunnel proximal to the translocon central pore and minimizing exposure of the TMD to cytosol. Insertases can also mediate TMD insertion into the lipid bilayer. Insertases include the bacterial YidC, mitochondrial Oxa1, and chloroplast Alb3, all of which are evolutionarily related. The conserved Hrd1 and Derlin enzyme families are examples of membrane bound quality control factors.
Examples
* Tetraspanins have 4 conserved transmembrane domains. * Mildew locus o (''mlo'') proteins have 7 conserved transmembrane domains that encode alpha helices.References
{{reflist Transmembrane proteins Protein structural motifs