Supramolecular polymer on:
[Wikipedia]
[Google]
[Amazon]
The term "
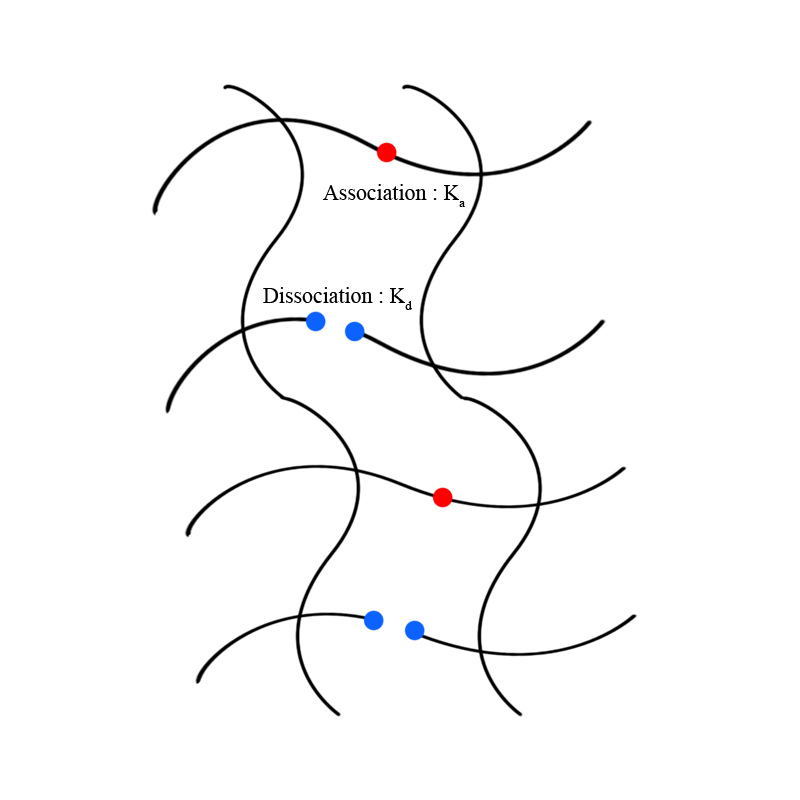 # Basic Principle : Noncovalent interactions between polymer molecules significantly affect the mechanical properties of supramolecular polymers. More interaction between polymers tends to enhance the interaction strength between polymers. The association rate and dissociation rate of interacting groups in polymer molecules determine intermolecular interaction strength. For supramolecular polymers, the dissociation kinetics for dynamic networks plays a critical role in the material design and mechanical properties of the SPNs(supramolecular polymer networks). By changing the dissociation rate of polymer crosslink dynamics, supramolecular polymers have adjustable mechanical properties. With a slow dissociation rate for dynamic networks of supramolecular polymers, glass-like mechanical properties are dominant, on the other hand, rubber-like mechanical properties are dominant for a fast dissociation rate. These properties can be obtained by changing the molecular structure of the crosslink part of the molecule.
# Experimental examples : One research controlled the molecular design of cucurbit ril, CB The hydrophobic structure of the second guest of CB-mediated host-guest interaction within its molecular structure can tune the dissociative kinetics of the dynamic crosslinks. To slow the dissociation rate (kd), a stronger enthalpic driving force is needed for the second guest association (ka) to release more of the conformationally restricted water from the CB(8] cavity. In other words, the hydrophobic second guest exhibited the highest Keq and lowest kd values. Therefore, by polymerizing different concentrations of polymer subgroups, different dynamics of the intermolecular network can be designed.For example, mechanical properties like compressive strain can be tuned by this process. Polymerized with different hydrophobic subgroups in CB The compressive strength was found to increase across the series in correlation with a decrease of kd, which could be tuned between 10–100MPa. NVI, is the most hydrophobic subgroup structure of monomer which have two benzene rings, on the other hand, BVI is the least hydrophobic subgroup structure of monomer via control group. Besides, varying concentrations of hydrophobic subgroups in CB polymerized molecules show different compressive properties. Polymers with the highest concentration of hydrophobic subgroups show the highest compressive strain and vice versa.
# Basic Principle : Noncovalent interactions between polymer molecules significantly affect the mechanical properties of supramolecular polymers. More interaction between polymers tends to enhance the interaction strength between polymers. The association rate and dissociation rate of interacting groups in polymer molecules determine intermolecular interaction strength. For supramolecular polymers, the dissociation kinetics for dynamic networks plays a critical role in the material design and mechanical properties of the SPNs(supramolecular polymer networks). By changing the dissociation rate of polymer crosslink dynamics, supramolecular polymers have adjustable mechanical properties. With a slow dissociation rate for dynamic networks of supramolecular polymers, glass-like mechanical properties are dominant, on the other hand, rubber-like mechanical properties are dominant for a fast dissociation rate. These properties can be obtained by changing the molecular structure of the crosslink part of the molecule.
# Experimental examples : One research controlled the molecular design of cucurbit ril, CB The hydrophobic structure of the second guest of CB-mediated host-guest interaction within its molecular structure can tune the dissociative kinetics of the dynamic crosslinks. To slow the dissociation rate (kd), a stronger enthalpic driving force is needed for the second guest association (ka) to release more of the conformationally restricted water from the CB(8] cavity. In other words, the hydrophobic second guest exhibited the highest Keq and lowest kd values. Therefore, by polymerizing different concentrations of polymer subgroups, different dynamics of the intermolecular network can be designed.For example, mechanical properties like compressive strain can be tuned by this process. Polymerized with different hydrophobic subgroups in CB The compressive strength was found to increase across the series in correlation with a decrease of kd, which could be tuned between 10–100MPa. NVI, is the most hydrophobic subgroup structure of monomer which have two benzene rings, on the other hand, BVI is the least hydrophobic subgroup structure of monomer via control group. Besides, varying concentrations of hydrophobic subgroups in CB polymerized molecules show different compressive properties. Polymers with the highest concentration of hydrophobic subgroups show the highest compressive strain and vice versa.
polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
" refers to large molecules whose structure is composed of multiple repeating units and the prefix "supra" meaning "beyond the limits of". Supramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
polymers are a new category of polymers that can potentially be used for material applications beyond the limits of conventional polymers. By definition, supramolecular polymers are polymeric arrays of monomeric units that are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bond A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen ...
ing, chalcogen bonding, and host–guest interaction. The direction and strength of the interactions are precisely tuned so that the array of molecules behaves as a polymer (that is, it behaves in a way that can be described by the theories of polymer physics) in dilute and concentrated solution, as well as in the bulk.
In conventional polymers, monomeric units are linked by strong covalent bonds and have excellent properties as materials; however, high temperatures and pressures are typically required for processing due to polymer entanglement in the highly viscous melt. Supramolecular polymers combine good material properties with low-viscosity melts that are easy to handle. Additionally, some supramolecular polymers have unique characteristics, such as the ability to self-heal
''Prunella'' is a genus of herbaceous plants in the family Lamiaceae, also known as self-heals, heal-all, or allheal for their use in herbal medicine.
Habitat
Most are native to Europe, Asia, and North Africa, but ''Prunella vulgaris'' (common ...
fractures. Although covalent polymers can be recycled, their strong covalent bonds never disintegrate, and go on to negatively affect the environment as plastic wastes. Thus, supramolecular polymers are increasingly getting attention because of their potential for the design of responsive, adaptive, self-healing, and environmentally friendly materials.
History
Modern concept of polymers credited to Hermann Staudinger, who substantiated the existence of covalently linked ultralong molecules in 1920, which he called as macromolecules. The preamble of the field of supramolecular polymers can be considered dye-aggregates and host-guest complexes. In early 19thcentury, scientists working in the field of pigments have noticed certain dye aggregates that may formed via "a special kind of polymerization", however no theory was proposed. After the establishment of the field of supramolecular chemistry and after the award of the Nobel Prize in chemistry in 1987 to Donald J. Cram,Jean-Marie Lehn
Jean-Marie Lehn (born 30 September 1939) is a French chemist. He received the Nobel Prize in Chemistry together with Donald Cram and Charles Pedersen in 1987 for his synthesis of cryptands. Lehn was an early innovator in the field of supramole ...
, and Charles J. Pedersen, chemists started to design and study larger assembled structures from small molecules. In 1988, Takuzo Aida, a Japanese polymer chemist, reported the concept of cofacial assembly wherein the amphiphilic porphyrin monomers are connected via van der Waals interaction forming one-dimensional architectures in solution, which can be considered as a prototype of supramolecular polymers. In the same year 1988, James D. Wuest introduced one-dimensional aggregates based on hydrogen bonding interaction in the crystalline state. With a different strategyusing hydrogen bonds, Jean M. J. Fréchet showed in 1989 that mesogenic molecules with carboxylic acid and pyridyl motifs, upon mixing in bulk, heterotropically dimerize to form a stable liquid crystalline structure. In 1990, Jean-Marie Lehn showed that this strategy can be expanded to form a new category of polymers, which he called "liquid crystalline supramolecular polymer" using complementary triple hydrogen bonding motifs in bulk. In 1993, M. Reza Ghadiri reported a nanotubular supramolecular polymer where a ''b''-sheet-forming macrocyclic peptide monomer assembled together via multiple hydrogen bonding between adjacent macrocycles. In 1994, Anselm. C. Griffin showed an amorphous supramolecular material using a single hydrogen bond between a homotropic molecules having carboxylic acid and pyridine termini. The idea to make mechanically strong polymeric materials by 1D supramolecular association of small molecules requires a high association constant between the repeating building blocks. In 1997, E.W. "Bert" Meijer reported a telechelic monomer with ureidopyrimidinone termini as a "self-complementary" quadruple hydrogen bonding motif and demonstrated that the resulting supramolecular polymer in chloroform shows a temperature-dependent viscoelastic property in solution. This is the first demonstration that supramolecular polymers, when sufficiently mechanically robust, are physically entangled in solution.
Formation mechanisms
Monomers undergoing supramolecular polymerization are considered to be in equilibrium with the growing polymers, and thermodynamic factors therefore dominate the system. However, when the constituent monomers are connected via strong and multivalent interactions, a "metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
" kinetic state can dominate the polymerization. An externally supplied energy, in the form of heat in most cases, can transform the "metastable" state into a thermodynamically stable polymer. A clear understanding of multiple pathways exist in supramolecular polymerization is still under debate, however, the concept of "pathway complexity", introduced by E.W. "Bert" Meijer, shed a light on the kinetic behavior of supramolecular polymerization. Thereafter, many dedicated scientists are expanding the scope of "pathway complexity" because it can produce a variety of interesting assembled structures from the same monomeric units. Along this line of kinetically controlled processes, supramolecular polymers having "stimuli-responsive" and "thermally bisignate" characteristics is also possible.
In conventional covalent polymerization, two models based on step-growth and chain-growth mechanisms are operative. Nowadays, a similar subdivision is acceptable for supramolecular polymerization; isodesmic also known as equal-K model (step-growth mechanism) and cooperative or nucleation-elongation model (chain-growth mechanism). A third category is seeded supramolecular polymerization, which can be considered as a special case of chain-growth mechanism.
Step-growth polymerization
Supramolecular equivalent of step-growth mechanism is commonly known as isodesmic or equal-K model (K represents the total binding interaction between two neighboring monomers). In isodesmic supramolecular polymerization, no critical temperature or concentration of monomers is required for the polymerization to occur and the association constant between polymer and monomer is independent of the polymer chain length. Instead, the length of the supramolecular polymer chains rises as the concentration of monomers in the solution increases, or as the temperature decreases. In conventional polycondensation, the association constant is usually large that leads to a high degree of polymerization; however, a byproduct is observed. In isodesmic supramolecular polymerization, due to non-covalent bonding, the association between monomeric units is weak, and the degree of polymerization strongly depends on the strength of interaction, i.e. multivalent interaction between monomeric units. For instance, supramolecular polymers consisting of bifunctional monomers having single hydrogen bonding donor/acceptor at their termini usually end up with low degree of polymerization, however those with quadrupole hydrogen bonding, as in the case of ureidopyrimidinone motifs, result in a high degree of polymerization. In ureidopyrimidinone-based supramolecular polymer, the experimentally observed molecular weight at semi-dilute concentrations is in the order of 106 Dalton and the molecular weight of the polymer can be controlled by adding mono-functional chain-cappers.Chain-growth polymerization
Conventional chain-growth polymerization involves at least two phases; initiation and propagation, while and in some cases termination and chain transfer phases also occur. Chain-growth supramolecular polymerization in a broad sense involves two distinct phases; a less favored nucleation and a favored propagation. In this mechanism, after the formation of a nucleus of a certain size, the association constant is increased, and further monomer addition becomes more favored, at which point the polymer growth is initiated. Long polymer chains will form only above a minimum concentration of monomer and below a certain temperature. However, to realize a covalent analogue of chain-growth supramolecular polymerization, a challenging prerequisite is the design of appropriate monomers that can polymerize only by the action of initiators. Recently one example of chain-growth supramolecular polymerization with "living" characteristics is demonstrated. In this case, a bowl-shaped monomer with amide-appended side chains form a kinetically favored intramolecular hydrogen bonding network and does not spontaneously undergo supramolecular polymerization at ambient temperatures. However, an N-methylated version of the monomer serves as an initiator by opening the intramolecular hydrogen bonding network for the supramolecular polymerization, just like ring-opening covalent polymerization. The chain end in this case remains active for further extension of supramolecular polymer and hence chain-growth mechanism allows for the precise control of supramolecular polymer materials.Seeded polymerization
This is a special category of chain-growth supramolecular polymerization, where the monomer nucleates only in an early stage of polymerization to generate "seeds" and becomes active for polymer chain elongation upon further addition of a new batch of monomer. A secondary nucleation is suppressed in most of the case and thus possible to realize a narrow polydispersity of the resulting supramolecular polymer. In 2007, Ian Manners and Mitchell A. Winnik introduced this concept using a polyferrocenyldimethylsilane–polyisoprene diblock copolymer as the monomer, which assembles into cylindrical micelles. When a fresh feed of the monomer is added to the micellar "seeds" obtained by sonication, the polymerization starts in a living polymerization manner. They named this method as crystallization-driven self-assembly (CDSA) and is applicable to construct micron-scale supramolecular anisotropic structures in 1D–3D. A conceptually different seeded supramolecular polymerization was shown by Kazunori Sugiyasu in a porphyrin-based monomer bearing amide-appended long alkyl chains. At low temperature, this monomer preferentially forms sphericalJ-aggregate
A J-aggregate is a type of dye with an absorption band that shifts to a longer wavelength (bathochromic shift) of increasing sharpness (higher absorption coefficient) when it aggregates under the influence of a solvent or additive or concentrati ...
s while fibrous H-aggregates at higher temperature. By adding a sonicated mixture of the J-aggregates ("seeds") into a concentrated solution of the J-aggregate particles, long fibers can be prepared via living seeded supramolecular polymerization. Frank Würthner achieved similar seeded supramolecular polymerization of amide functionalized perylene bisimide as monomer. Importantly, the seeded supramolecular polymerization is also applicable to prepare supramolecular block copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
.
Examples
Hydrogen bonding interaction
Monomers capable of forming single, double, triple or quadruple hydrogen bonding has been utilized for making supramolecular polymers, and increased association of monomers obviously possible when monomers have maximum number of hydrogen bonding donor/acceptor motifs. For instance, ureidopyrimidinone-based monomer with self-complementary quadruple hydrogen bonding termini polymerized in solution, accordingly with the theory of conventional polymers and displayed a distinct viscoelastic nature at ambient temperatures.π-π stacking
Monomers with aromatic motifs such as bis(merocyanine), oligo(''para''-phenylenevinylene) (OPV), perylene bisimide (PBI) dye, cyanine dye, corannulene and nano-graphene derivatives have been employed to prepare supramolecular polymers. In some cases, hydrogen bonding side chains appended onto the core aromatic motif help to hold the monomer strongly in the supramolecular polymer. A notable system in this category is a nanotubular supramolecular polymer formed by the supramolecular polymerization of amphiphilic hexa-''peri''- hexabenzocoronene (HBC) derivatives. Generally, nanotubes are categorized as 1D objects morphologically, however, their walls adopt a 2D geometry and therefore require a different design strategy. HBC amphiphiles in polar solvents solvophobically assemble into a 2D bilayer membrane, which roles up into a helical tape or a nanotubular polymer. Conceptually similar amphiphilic design based oncyanine dye
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic ...
and zinc chlorin dye also polymerize in water resulting in nanotubular supramolecular polymers.
Host-guest interaction
A variety of supramolecular polymers can be synthesized by using monomers with host-guest complementary binding motifs, such ascrown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
s/ammonium ions, cucurbituril
In host-guest chemistry, cucurbiturils are macrocyclic molecules made of glycoluril () monomers linked by methylene bridges (). The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity ( ...
s/viologen
Viologens are organic compounds with the formula (C5H4NR)2n+. In some viologens, the pyridyl groups are further modified.
Viologens are called so, because these compounds produce violet color on reduction iolet + Latin ''gen'', generator of
T ...
s, calixarene
A calixarene is a macrocycle or cyclic oligomer based on a methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry.
Nomenclature
Cal ...
/viologens, cyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical ...
s/adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the ...
derivatives, and pillar arene/imidazolium derivatives 0–33 When the monomers are "heteroditopic", supramolecular copolymers results, provided the monomers does not homopolymerize. Akira Harada was one of the firstwhorecognize the importance of combining polymers and cyclodextrins. Feihe Huang showed an example of supramolecular alternating copolymer from two heteroditopic monomers carrying both crown ether and ammonium ion termini. Takeharo Haino demonstrated an extreme example of sequence control in supramolecular copolymer, where three heteroditopic monomers are arranged in an ABC sequence along the copolymer chain. The design strategy utilizing three distinct binding interactions; ball-and-socket (calix rene/C60), donor-acceptor (bisporphyrin/trinitrofluorenone), and Hamilton's H-bonding interactions is the key to attain a high orthogonality to form an ABC supramolecular terpolymer.
Chirality
Stereochemical information of achiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
monomer can be expressed in a supramolecular polymer. Helical supramolecular polymer with P-and M-conformation are widely seen, especially those composed of disc-shaped monomers. When the monomers are achiral, both P-and M-helices are formed in equal amounts. When the monomers are chiral, typically due to the presence of one or more stereocenters in the side chains, the diastereomeric relationship between P- and M-helices leads to the preference of one conformation over the other. Typical example is a C3-symmetric disk-shaped chiral monomer that forms helical supramolecular polymers via the "majority rule". A slight excess of one enantiomer of the chiral monomer resulted in a strong bias to either the right-handed or left-handed helical geometry at the supramolecular polymer level. In this case, a characteristic nonlinear dependence of the anisotropic factor, g, on the enantiomeric excess of a chiral monomer can be generally observed. Like in small molecule based chiral system, chirality of a supramolecular polymer also affected by chiral solvents. Some application such as a catalyst for asymmetric synthesis and circular polarized luminescence are observed in chiral supramolecular polymers too.
Copolymers
Acopolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
is formed from more than one monomeric species. Advanced polymerization techniques have been established for the preparation of covalent copolymers, however supramolecular copolymers are still in its infancy and is slowly progressing. In recent years, all plausible category of supramolecular copolymers such as random, alternating, block, blocky, or periodic has been demonstrated in a broad sense.
Properties
In the last 30 years, the field of supramolecular polymers has grown into a very important new branch of polymer science. It has attracted numerous research activities in academia and industrial laboratories worldwide. New dynamic materials with a variety of anomalous properties are added to the field of materials engineering. Many applications in sustainability (easy processing and recycling), electronics, and medicine as well as cosmetics have become available.Reversibility and dynamicity
One of the important properties of supramolecular polymers is their reversible interactions in the monomeric array. When the interaction between monomers are sufficiently strong, interesting material properties can be expected. The thermodynamic stability of a supramolecular polymer can be described using the association constant, Kass. When Kass ≤ 104M−1, the polymeric aggregates are typically small in size and do not show any interesting properties and when Kass≥ 1010 M−1, the supramolecular polymer behaves just like covalent polymers due to the lack of dynamics. So, an optimum Kass = 104–1010M−1need to be attained for producing functional supramolecular polymers. The dynamics and stability of the supramolecular polymers often affect by the influence of additives (e.g. co-solvent or chain-capper). When a good solvent, for instance chloroform, is added to a supramolecular polymer in a poor solvent, for instance heptane, the polymer disassembles. However, in some cases, cosolvents contribute the stabilization/destabilization of supramolecular polymer. For instance, supramolecular polymerization of a hydrogen bonding porphyrin-based monomer in a hydrocarbon solvent containing a minute amount of a hydrogen bond scavenging alcohol shows distinct pathways, i.e. polymerization favored both by cooling as well as heating, and is known as "thermally bisignate supramolecular polymerization". In another example, minute amounts of molecularly dissolved water molecules in apolar solvents, like methylcyclohexane, become part of the supramolecular polymer at lower temperatures, due to specific hydrogen bonding interaction between the monomer and water.Self-healing
One of the fascinating properties of supramolecular polymers is its ability to self-heal upon fracture occur. A supramolecular rubber based on vitrimers, introduced by Ludwik Leibler, can self-heal simply by pressing the two broken edges of the material together. In this case, fractures occur when hydrogen bonds between monomers in the material are broken; bringing the edges of the fracture together allows the hydrogen bonds to re-form, sealing up the gap. Impressively, the dynamic behavior of the hydrogen bonds does not compromise the properties of the material. High mechanical strength of a material and self-healing ability is generally mutually exclusive. Thus, a glassy material that can self-heal at room temperature remained a challenge until recently. In an elegant design, Takuzo Aida introduced an innovative polymer glass composed of a supramolecularly polymerized oligomeric ether thiourea, which is mechanically robust (''e''= 1.4 GPa) but can self-heal, even at room temperature, just by a compression at the fractured surfaces. The invention of self-healable polymer glass updated the preconception that only soft rubbery materials can heal. Another strategy uses a bivalent poly(isobutylene)s (PIBs) withbarbituric acid
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid i ...
functionalized at head and tail. Multiple hydrogen bonding existed between the carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
and amide group of barbituric acid enable it to form a supramolecular network. In this case, the snipped small PIBs-based disks can recover itself from mechanical damage after several-hour contact at room temperature.
Covalent polymers containing coordination complexes also have studied for making self-healing materials. Taking advantage of coordination interactions between catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
and ferric ions, researchers developed pH-controlled self-healing supramolecular polymers. The formation of mono-, bis- and triscatehchol-Fe3+ complexes can be manipulated by pH, of which the bis- and triscatehchol-Fe3+ complexes show elastic moduli as well as self-healing capacity. For example, the triscatehchol-Fe3+ can restore its cohesiveness and shape after being torn. Chain-folding polyimide and pyrenyl-end-capped chains give rise to supramolecular networks.
Optoelectronic
To achieve the light-to-charge conversion is the prerequisite step in artificialphotosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
systems. By incorporating electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chemi ...
s and electron acceptors into the supramolecular polymers, a number of artificial systems, including photosynthesis system, can be constructed. Owing to the existence of more than one interactions (π-π interaction, hydrogen bonding interaction and the like), electron donor and electron acceptor can be held together in a proper proximity to afford long-lived charge separated states. Then a light-to-charge conversion system with faster photoinduced electron transfer and higher electron-transfer efficiency can be achieved in these artificial polymers.
Biocompatible
It is quite common that biomolecules, such as DNA,protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
and the like, come into being through various noncovalent interactions in biological system
A biological system is a complex network which connects several biologically relevant entities. Biological organization spans several scales and are determined based different structures depending on what the system is. Examples of biological syst ...
. Likewise, supramolecular polymers assembles themself via a combination of noncovalent interactions. Such formation manner endows supramolecular polymers with features, being more sensitive to external stimuli and able to render reversibly dynamic changes in structures and functions. By modifying monomeric units of supramolecular polymers with water-soluble pendants, bioactive moieties as well as biomarkers, supramolecular polymers can realize various kinds of functions and applications in biomedical field. At the same time, their reversible and dynamic nature make supramolecular polymers bio-degradable, which surmounts hard-to-degrade issue of covalent polymers and makes supramolecular polymers a promising platform for biomedical applications. Being able to degrade in biological environment lowers potential toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
of polymers to a great extent and therefore, enhances biocompatibility of supramolecular polymers.
Biomedical applications
With the excellent nature inbiodegradation
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
and biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing de ...
, supramolecular polymers show great potential in the development of drug delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to dr ...
, gene transfection
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. It may also refer to other methods and cell types, although other terms are often preferred: " transformation" is typically used to des ...
and other biomedical applications.
Drug delivery: Multiple cellular stimuli
A stimulus is something that causes a physiological response. It may refer to:
* Stimulation
** Stimulus (physiology), something external that influences an activity
** Stimulus (psychology), a concept in behaviorism and perception
* Stimulus (eco ...
could induce responses in supramolecular polymers. The dynamic molecular skeletons of supramolecular polymers can be depolymerized when exposing to the external stimuli like pH ''in vivo''. On the basis of this property, supramolecular polymers are capable of being a drug carrier. Making use of hydrogen bonding between nucleobases to induce self-assemble into pH-sensitive spherical micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated coll ...
s.
Gene transfection: Effective and low-toxic nonviral cationic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
vectors are highly desired in the field of gene therapy. On account of the dynamic and stimuli-responsive properties, supramolecular polymers offer a cogent platform to construct vectors for gene transfection. By combining ferrocene dimer with β-cyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical ...
dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
, a redox-control supramolecular polymers system has been proposed as a vector. In COS-7 cells, this supramolecular polymersic vector can release enclosed DNA upon exposing to hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
and achieve gene transfection.
Adjustable mechanical properties
 # Basic Principle : Noncovalent interactions between polymer molecules significantly affect the mechanical properties of supramolecular polymers. More interaction between polymers tends to enhance the interaction strength between polymers. The association rate and dissociation rate of interacting groups in polymer molecules determine intermolecular interaction strength. For supramolecular polymers, the dissociation kinetics for dynamic networks plays a critical role in the material design and mechanical properties of the SPNs(supramolecular polymer networks). By changing the dissociation rate of polymer crosslink dynamics, supramolecular polymers have adjustable mechanical properties. With a slow dissociation rate for dynamic networks of supramolecular polymers, glass-like mechanical properties are dominant, on the other hand, rubber-like mechanical properties are dominant for a fast dissociation rate. These properties can be obtained by changing the molecular structure of the crosslink part of the molecule.
# Experimental examples : One research controlled the molecular design of cucurbit ril, CB The hydrophobic structure of the second guest of CB-mediated host-guest interaction within its molecular structure can tune the dissociative kinetics of the dynamic crosslinks. To slow the dissociation rate (kd), a stronger enthalpic driving force is needed for the second guest association (ka) to release more of the conformationally restricted water from the CB(8] cavity. In other words, the hydrophobic second guest exhibited the highest Keq and lowest kd values. Therefore, by polymerizing different concentrations of polymer subgroups, different dynamics of the intermolecular network can be designed.For example, mechanical properties like compressive strain can be tuned by this process. Polymerized with different hydrophobic subgroups in CB The compressive strength was found to increase across the series in correlation with a decrease of kd, which could be tuned between 10–100MPa. NVI, is the most hydrophobic subgroup structure of monomer which have two benzene rings, on the other hand, BVI is the least hydrophobic subgroup structure of monomer via control group. Besides, varying concentrations of hydrophobic subgroups in CB polymerized molecules show different compressive properties. Polymers with the highest concentration of hydrophobic subgroups show the highest compressive strain and vice versa.
# Basic Principle : Noncovalent interactions between polymer molecules significantly affect the mechanical properties of supramolecular polymers. More interaction between polymers tends to enhance the interaction strength between polymers. The association rate and dissociation rate of interacting groups in polymer molecules determine intermolecular interaction strength. For supramolecular polymers, the dissociation kinetics for dynamic networks plays a critical role in the material design and mechanical properties of the SPNs(supramolecular polymer networks). By changing the dissociation rate of polymer crosslink dynamics, supramolecular polymers have adjustable mechanical properties. With a slow dissociation rate for dynamic networks of supramolecular polymers, glass-like mechanical properties are dominant, on the other hand, rubber-like mechanical properties are dominant for a fast dissociation rate. These properties can be obtained by changing the molecular structure of the crosslink part of the molecule.
# Experimental examples : One research controlled the molecular design of cucurbit ril, CB The hydrophobic structure of the second guest of CB-mediated host-guest interaction within its molecular structure can tune the dissociative kinetics of the dynamic crosslinks. To slow the dissociation rate (kd), a stronger enthalpic driving force is needed for the second guest association (ka) to release more of the conformationally restricted water from the CB(8] cavity. In other words, the hydrophobic second guest exhibited the highest Keq and lowest kd values. Therefore, by polymerizing different concentrations of polymer subgroups, different dynamics of the intermolecular network can be designed.For example, mechanical properties like compressive strain can be tuned by this process. Polymerized with different hydrophobic subgroups in CB The compressive strength was found to increase across the series in correlation with a decrease of kd, which could be tuned between 10–100MPa. NVI, is the most hydrophobic subgroup structure of monomer which have two benzene rings, on the other hand, BVI is the least hydrophobic subgroup structure of monomer via control group. Besides, varying concentrations of hydrophobic subgroups in CB polymerized molecules show different compressive properties. Polymers with the highest concentration of hydrophobic subgroups show the highest compressive strain and vice versa.
Biomaterials
Supramolecular polymers with specific, directional, tunable and reversible non-covalent interactions should be advantageous for biomaterials as well as biomedical applications. For instance, the reversible nature of supramolecular polymers can produce biomaterials that can sense and respond to physiological cues, or that mimic the structural and functional aspects of biological signaling. On the basis of their formation mechanisms, supramolecular biomaterials can be broadly classified as: (1) materials prepared from one-dimensional assemblies of molecular stacking motifs as in the case of peptide amphiphiles introduced by Samuel I. Stupp, and (2) materials prepared through chain extension of oligomers or through crosslinking of polymeric precursors by specific supramolecular recognition motifs. Rationally designed supramolecular polymers-based polymers can simultaneously meet the requirements of aqueous compatibility, bio-degradability, biocompatibility, stimuli-responsiveness and other strict criterion. Consequently, supramolecular polymers can be applied to the biomedical field as a robust system. Other than applications mentioned above, other important and fascinating biomedical applications, like protein delivery, bio-imaging
Imaging is the representation or reproduction of an object's form; especially a visual representation (i.e., the formation of an image).
Imaging technology is the application of materials and methods to create, preserve, or duplicate images.
...
and diagnosis
Diagnosis is the identification of the nature and cause of a certain phenomenon. Diagnosis is used in many different disciplines, with variations in the use of logic, analytics, and experience, to determine " cause and effect". In systems engin ...
and tissue engineering
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biolog ...
, are also well developed.
Conceptual expansion
Unconventional monomers
Over the time, methods for supramolecular polymerization has expanded, and the range of its useable monomers has diversified. In addition to plethora of molecular motifs, biomolecules such as DNA, DNA nanostructures and proteins as well as inorganic objects as unconventional monomers has recently been investigated for supramolecular polymerization. In all of these cases, monomers are in much higher size, usually several nanometers, and the non-covalent interactions varies from hydrogen bonding, host-guest and metal coordination. A notable example is Mg2+-assisted multivalent supramolecular polymerization of ATP-responsive biomolecular machines, chaperonine GroEL, resulting in a highly stable protein nanotube. Importantly, this nanotube shows an ATPase activity and dissociates into short-chain oligomers when treated with ATP because of the opening/closing motions of the constituent GroEL units.Unconventional media
Supramolecular polymers usually prepared in solution. However anomalous polymeric properties can be expected when these polymers are prepared without a conventional organic or aqueous medium. For instance, liquid crystal media may affect the elementary steps of supramolecular polymerization as demonstrated by Takashi Kato in 1998, in the supramolecular crosslinking polymerization of physical gelators, which form a liquid crystal physical gel. When monomers are designed to be highly affinitive toward the LC media, supramolecular polymerization causes an order-increasing phase transition, resulting in a core-shell columnar LC. Supramolecular polymers can also be prepared in the solid-state, for instance, a nucleobase-appended telechelic oligomer as a monomer, resulted in the formation of 1D fibers upon cooling from its hot melt. As a new class of materials, supramolecular polymers formed at electrode and at the interface also become available.References
{{DEFAULTSORT:Supramolecular Polymers Supramolecular chemistry Polymers