|
Barbituric Acid
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active. The compound was first synthesised by Adolf von Baeyer. Naming It remains unclear why Baeyer chose to name the compound that he discovered "barbituric acid". In his textbook ''Organic Chemistry'', the American organic chemist Louis Frederick Fieser (1899–1977) initially speculated that the name stemmed from the German word ''Schlüsselbart'' (literally, the beard (''Bart Latin: ''barba'') of a key (''Schlüssel'')' that is, the bit of a key), because Baeyer had regarded barbituric acid as central (or "key") to understanding uric acid and its derivatives. However, Fieser subsequently decided that Baeyer had named the compound after a young lady whom he had met and who was called "Barbara"' hence th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Press
The CRC Press, LLC is an American publishing group that specializes in producing technical books. Many of their books relate to engineering, science and mathematics. Their scope also includes books on business, forensics and information technology. CRC Press is now a division of Taylor & Francis, itself a subsidiary of Informa. History The CRC Press was founded as the Chemical Rubber Company (CRC) in 1903 by brothers Arthur, Leo and Emanuel Friedman in Cleveland, Ohio, based on an earlier enterprise by Arthur, who had begun selling rubber laboratory aprons in 1900. The company gradually expanded to include sales of laboratory equipment to chemists. In 1913 the CRC offered a short (116-page) manual called the ''Rubber Handbook'' as an incentive for any purchase of a dozen aprons. Since then the ''Rubber Handbook'' has evolved into the CRC's flagship book, the ''CRC Handbook of Chemistry and Physics''. In 1964, Chemical Rubber decided to focus on its publishing ventures, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
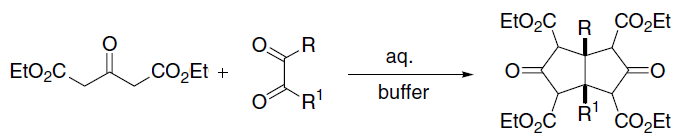
Diethyl Malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6. Structure and properties Malonic acid is a rather simple dicarboxylic acid, with two the carboxyl groups close together. In forming diethyl malonate from malonic acid, the hydroxyl group (−OH) on both of the carboxyl groups is replaced by an ethoxy group (−OEt; −OCH2CH3). The methylene group (−CH2−) in the middle of the malonic part of the diethyl malonate molecule is neighboured by two carbonyl groups (−C(=O)−). The hydrogen atoms on the carbon adjacent to the carbonyl group in a molecule is significantly more acidic than hydrogen atoms on a carbon adjacent to alkyl groups (up to 30 orders of magnitude). (This is known as the α position with res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Building Block (chemistry)
Building block is a term in chemistry which is used to describe a virtual molecular fragment or a real chemical compound the molecules of which possess reactive functional groups. Building blocks are used for bottom-up modular assembly of molecular architectures: nano-particles, metal-organic frameworks, organic molecular constructs, supra-molecular complexes. Using building blocks ensures strict control of what a final compound or a (supra)molecular construct will be. Building blocks for medicinal chemistry In medicinal chemistry, the term defines either imaginable, virtual molecular fragments or chemical reagents from which drugs or drug candidates might be constructed or synthetically prepared. Virtual building blocks Virtual building blocks are used in drug discovery for drug design and virtual screening, addressing the desire to have controllable molecular morphologies that interact with biological targets. Of special interest for this purpose are the building blocks co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenobarbital
Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. In developed countries it is used for veterinary purposes. It may be used intravenously, injected into a muscle, or taken by mouth. The injectable form may be used to treat status epilepticus. Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery. It usually begins working within five minutes when used intravenously and half an hour when administered by mouth. Its effects last for between four hours and two days. Side effects include a decreased level of consciousness along with a decreased e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barbital
Barbital (or barbitone), marketed under the brand names Veronal for the pure acid and Medinal for the sodium salt, was the first commercially available barbiturate. It was used as a sleeping aid (hypnotic) from 1903 until the mid-1950s. The chemical names for barbital are diethylmalonyl urea or diethylbarbituric acid; hence, the sodium salt (known as medinal, a genericised trademark in the United Kingdom) is known also as sodium diethylbarbiturate. Synthesis Barbital, then called "Veronal", was first synthesized in 1902 by German chemists Emil Fischer and Joseph von Mering, who published their discovery in 1903. Barbital was prepared by condensing diethylmalonic ester with urea in the presence of sodium ethoxide, or by adding at least two molar equivalents of ethyl iodide to the silver salt of malonylurea (barbituric acid) or possibly to a basic solution of the acid. The result was an odorless, slightly bitter, white crystalline powder. Its introduction followed the investi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System Depressant
A depressant, or central depressant, is a drug that lowers neurotransmission levels, which is to depress or reduce arousal or stimulation, in various areas of the brain. Depressants are also colloquially referred to as downers as they lower the level of arousal when taken. Stimulants or "uppers" increase mental or physical function, hence the opposite drug class of depressants is stimulants, not antidepressants. Depressants are widely used throughout the world as prescription medicines and as illicit substances. Alcohol is a very prominent depressant. Alcohol can be and is more likely to be a large problem among teenagers and young adults. When depressants are used, effects often include ataxia, anxiolysis, pain relief, sedation or somnolence, and cognitive or memory impairment, as well as in some instances, euphoria, dissociation, muscle relaxation, lowered blood pressure or heart rate, respiratory depression, and anticonvulsant effects. Depressants also act to produce anesth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoevenagel Condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence '' condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the alde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds. Properties Tautomerism The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimedone
Dimedone is a cyclic diketone used in organic chemistry to determine whether a compound contains an aldehyde group. Cyclohexanediones in general can be used as catalysts in the formation of transition-metal complexes. Other uses include applications in colorimetry, crystallography, luminescence and spectrophotometric analysis. It can also be used for chemistry involving organic compounds of low electrical resistance. Synthesis Dimedone is prepared from mesityl oxide and diethyl malonate. Physical properties Dimedone usually comes in the form of white crystals. It is stable under ambient conditions and soluble in water, as well as ethanol and methanol. It has a melting point range of 147–150 °C (420–423 K). Chemical properties Tautomerism Dimedone is in equilibrium with its tautomer Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomeriz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, di ketones, di esters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |