A supercritical fluid (SCF) is any substance at a
temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
and
pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
above its
critical point, where distinct
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
and
gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
phases do not exist, but below the pressure required to compress it into a
solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
. It can
effuse through porous solids like a gas, overcoming the
mass transfer
Mass transfer is the net movement of mass from one location (usually meaning stream, phase, fraction or component) to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtration, ...
limitations that slow liquid transport through such materials. SCF are much superior to gases in their ability to
dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in
density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
, allowing many properties of a supercritical fluid to be "fine-tuned".
Supercritical fluids occur in the
atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
s of the
gas giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" ...
s
Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but ...
and
Saturn
Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius of about nine and a half times that of Earth. It has only one-eighth the average density of Earth; h ...
, the
terrestrial planet
A terrestrial planet, telluric planet, or rocky planet, is a planet that is composed primarily of silicate rocks or metals. Within the Solar System, the terrestrial planets accepted by the IAU are the inner planets closest to the Sun: Mercury, Ve ...
Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never fa ...
, and probably in those of the
ice giant
An ice giant is a giant planet composed mainly of elements heavier than hydrogen and helium, such as oxygen, carbon, nitrogen, and sulfur. There are two ice giants in the Solar System: Uranus and Neptune.
In astrophysics and planetary science t ...
s
Uranus
Uranus is the seventh planet from the Sun. Its name is a reference to the Greek god of the sky, Uranus (mythology), Uranus (Caelus), who, according to Greek mythology, was the great-grandfather of Ares (Mars (mythology), Mars), grandfather ...
and
Neptune
Neptune is the eighth planet from the Sun and the farthest known planet in the Solar System. It is the fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 times ...
. Supercritical water is found on
Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surfa ...
, such as the water issuing from
black smokers, a type of underwater
hydrothermal vent
A hydrothermal vent is a fissure on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspot ...
. They are used as a substitute for
organic solvents in a range of industrial and laboratory processes.
Carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
are the most commonly used supercritical fluids; they are often used for
decaffeination
Decaffeination is the removal of caffeine from coffee beans, cocoa, tea leaves, and other caffeine-containing materials. Decaffeinated drinks contain typically 1–2% of the original caffeine content, and sometimes as much as 20%. Decaffeinate ...
and
power generation
Electricity generation is the process of generating electric power from sources of primary energy. For utilities in the electric power industry, it is the stage prior to its delivery ( transmission, distribution, etc.) to end users or its stor ...
, respectively. An interesting property is that some substances are soluble in the supercritical state of a solvent (e.g. carbon dioxide) but insoluble in the gaseous or liquid state—or vice versa. This can be used to extract a substance and transport it elsewhere in solution before depositing it in the desired place by simply allowing or inducing a
phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
in the solvent.
Properties
Supercritical fluids generally have properties between those of a gas and a liquid. In Table 1, the critical properties are shown for some substances that are commonly used as supercritical fluids.
†Source: International Association for Properties of Water and Steam
IAPWS
Table 2 shows density, diffusivity and viscosity for typical liquids, gases and supercritical fluids.
Also, there is no
surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
in a supercritical fluid, as there is no liquid/gas phase boundary. By changing the pressure and temperature of the fluid, the properties can be "tuned" to be more liquid-like or more gas-like. One of the most important properties is the solubility of material in the fluid. Solubility in a supercritical fluid tends to increase with density of the fluid (at constant temperature). Since density increases with pressure, solubility tends to increase with pressure. The relationship with temperature is a little more complicated. At constant density, solubility will increase with temperature. However, close to the critical point, the density can drop sharply with a slight increase in temperature. Therefore, close to the critical temperature, solubility often drops with increasing temperature, then rises again.
Mixtures
Typically, supercritical fluids are completely
miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies ...
with each other, so that a binary mixture forms a single gaseous phase if the critical point of the mixture is exceeded. However, exceptions are known in systems where one component is much more volatile than the other, which in some cases form two immiscible gas phases at high pressure and temperatures above the component critical points. This behavior has been found for example in the systems N
2-NH
3, NH
3-CH
4, SO
2-N
2 and n-butane-H
2O.
The critical point of a binary mixture can be estimated as the
arithmetic mean
In mathematics and statistics, the arithmetic mean ( ) or arithmetic average, or just the ''mean'' or the ''average'' (when the context is clear), is the sum of a collection of numbers divided by the count of numbers in the collection. The colle ...
of the critical temperatures and pressures of the two components,
where ''χ''
''i'' denotes the
mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ex ...
of component ''i''.
For greater accuracy, the critical point can be calculated using
equations of state, such as the
Peng–Robinson, or
group-contribution methods. Other properties, such as density, can also be calculated using equations of state.
Phase diagram

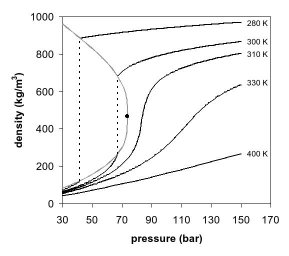
Figures 1 and 2 show two-dimensional projections of a
phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous ...
. In the pressure-temperature phase diagram (Fig. 1) the
boiling curve separates the
gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
and liquid region and ends in the critical point, where the liquid and gas phases disappear to become a single supercritical phase.
The appearance of a single phase can also be observed in the density-pressure phase diagram for carbon dioxide (Fig. 2). At well below the critical temperature, e.g., 280 K, as the pressure increases, the gas compresses and eventually (at just over 40
bar
Bar or BAR may refer to:
Food and drink
* Bar (establishment), selling alcoholic beverages
* Candy bar
* Chocolate bar
Science and technology
* Bar (river morphology), a deposit of sediment
* Bar (tropical cyclone), a layer of cloud
* Bar (u ...
) condenses into a much denser liquid, resulting in the discontinuity in the line (vertical dotted line). The system consists of 2 phases in
equilibrium, a dense liquid and a low density gas. As the critical temperature is approached (300 K), the density of the gas at equilibrium becomes higher, and that of the liquid lower. At the critical point, (304.1 K and 7.38 MPa (73.8 bar)), there is no difference in density, and the 2 phases become one fluid phase. Thus, above the critical temperature a gas cannot be liquefied by pressure. At slightly above the critical temperature (310 K), in the vicinity of the critical pressure, the line is almost vertical. A small increase in pressure causes a large increase in the density of the supercritical phase. Many other physical properties also show large gradients with pressure near the critical point, e.g.
viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
, the
relative permittivity
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulat ...
and the solvent strength, which are all closely related to the density. At higher temperatures, the fluid starts to behave more like an ideal gas, with a more linear density/pressure relationship, as can be seen in Figure 2. For carbon dioxide at 400 K, the density increases almost linearly with pressure.
Many pressurized gases are actually supercritical fluids. For example, nitrogen has a critical point of 126.2 K (−147 °C) and 3.4 MPa (34 bar). Therefore, nitrogen (or compressed air) in a gas cylinder above this pressure is actually a supercritical fluid. These are more often known as permanent gases. At room temperature, they are well above their critical temperature, and therefore behave as a nearly ideal gas, similar to CO
2 at 400 K above. However, they cannot be liquified by mechanical pressure unless cooled below their critical temperature, requiring gravitational pressure such as within
gas giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" ...
s to produce a liquid or solid at high temperatures. Above the critical temperature, elevated pressures can increase the density enough that the SCF exhibits liquid-like density and behaviour. At very high pressures, an SCF can be compressed into a solid because the melting curve extends to the right of the critical point in the P/T phase diagram. While the pressure required to compress supercritical CO
2 into a solid can be, depending on the temperature, as low as 570 MPa, that required to solidify supercritical water is 14,000 MPa.
The
Fisher–Widom line, the
Widom line, or the
Frenkel line
In fluid dynamics, the Frenkel line is a proposed boundary on the phase diagram of a supercritical fluid, separating regions of qualitatively different behavior. Fluids on opposite sides of the line have been described as "liquidlike" or "gaslik ...
are thermodynamic concepts that allow to distinguish liquid-like and gas-like states within the supercritical fluid.
In recent years, a significant effort has been devoted to investigation of various properties of supercritical fluids. This has been an exciting field with a long history since 1822 when Baron
Charles Cagniard de la Tour
Baron Charles Cagniard de la Tour (31 March 1777 – 5 July 1859) was a French engineer and physicist. Charles Cagniard was born in Paris, and after attending the École Polytechnique became one of the ''ingénieurs géographiques''. He examined t ...
discovered supercritical fluids while conducting experiments involving the discontinuities of the sound in a sealed gun barrel filled with various fluids at high temperature.
More recently, supercritical fluids have found application in a variety of fields, ranging from the extraction of floral fragrance from flowers to applications in food science such as creating decaffeinated coffee, functional food ingredients, pharmaceuticals, cosmetics, polymers, powders, bio- and functional materials, nano-systems, natural products, biotechnology, fossil and bio-fuels, microelectronics, energy and environment. Much of the excitement and interest of the past decade is due to the enormous progress made in increasing the power of relevant experimental tools. The development of new experimental methods and improvement of existing ones continues to play an important role in this field, with recent research focusing on dynamic properties of fluids.
Natural occurrence
Hydrothermal circulation

Hydrothermal circulation occurs within the Earth's crust wherever fluid becomes heated and begins to convect. These fluids are thought to reach supercritical conditions under a number of different settings, such as in the formation of porphyry copper deposits or high temperature circulation of seawater in the sea floor. At mid-ocean ridges, this circulation is most evident by the appearance of hydrothermal vents known as "black smokers". These are large (metres high) chimneys of sulfide and sulfate minerals which vent fluids up to 400 °C. The fluids appear like great black billowing clouds of smoke due to the precipitation of dissolved metals in the fluid. It is likely that at depth many of these vent sites reach supercritical conditions, but most cool sufficiently by the time they reach the sea floor to be subcritical. One particular vent site, Turtle Pits, has displayed a brief period of supercriticality at the vent site. A further site,
Beebe, in the Cayman Trough, is thought to display sustained supercriticality at the vent orifice.
Planetary atmospheres
The atmosphere of
Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never fa ...
is 96.5% carbon dioxide and 3.5% nitrogen. The surface pressure is 9.3 MPa (93 bar) and the surface temperature is 735 K, above the critical points of both major constituents and making the surface atmosphere a supercritical fluid.
The interior atmospheres of the solar system's
gas giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" ...
planets are composed mainly of hydrogen and helium at temperatures well above their critical points. The gaseous outer atmospheres of
Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but ...
and
Saturn
Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius of about nine and a half times that of Earth. It has only one-eighth the average density of Earth; h ...
transition smoothly into the dense liquid interior, while the nature of the transition zones of
Neptune
Neptune is the eighth planet from the Sun and the farthest known planet in the Solar System. It is the fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 times ...
and
Uranus
Uranus is the seventh planet from the Sun. Its name is a reference to the Greek god of the sky, Uranus (mythology), Uranus (Caelus), who, according to Greek mythology, was the great-grandfather of Ares (Mars (mythology), Mars), grandfather ...
is unknown. Theoretical models of
extrasolar planet
An exoplanet or extrasolar planet is a planet outside the Solar System. The first possible evidence of an exoplanet was noted in 1917 but was not recognized as such. The first confirmation of detection occurred in 1992. A different planet, init ...
Gliese 876 d
Gliese 876 d is an exoplanet approximately 15 light-years away in the constellation of Aquarius. The planet was the third planet discovered orbiting the red dwarf Gliese 876. It was the lowest-mass extrasolar planet apart from the pulsar planets o ...
have posited an ocean of pressurized, supercritical fluid water with a sheet of solid high pressure water ice at the bottom.
Applications
Supercritical fluid extraction
The advantages of
supercritical fluid extraction (compared with liquid extraction) are that it is relatively rapid because of the low viscosities and high diffusivities associated with supercritical fluids. Alternative solvents to supercritical fluids may be poisonous, flammable or an environmental harzard to a much larger extent than water or carbon dioxide are. The extraction can be selective to some extent by controlling the density of the medium, and the extracted material is easily recovered by simply depressurizing, allowing the supercritical fluid to return to gas phase and evaporate leaving little or no solvent residues. Carbon dioxide is the most common supercritical solvent. It is used on a large scale for the
decaffeination
Decaffeination is the removal of caffeine from coffee beans, cocoa, tea leaves, and other caffeine-containing materials. Decaffeinated drinks contain typically 1–2% of the original caffeine content, and sometimes as much as 20%. Decaffeinate ...
of green coffee beans, the extraction of
hops
Hops are the flowers (also called seed cones or strobiles) of the hop plant ''Humulus lupulus'', a member of the Cannabaceae family of flowering plants. They are used primarily as a bittering, flavouring, and stability agent in beer, to whi ...
for beer production, and the production of
essential oils
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the o ...
and pharmaceutical products from plants. A few
laboratory
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratory services are provided in a variety of settings: physicia ...
test method
A test method is a method for a test in science or engineering, such as a physical test, chemical test, or statistical test. It is a definitive procedure that produces a test result. In order to ensure accurate and relevant test results, a test met ...
s include the use of
supercritical fluid extraction as an extraction method instead of using traditional
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s.
Supercritical fluid decomposition
Supercritical water can be used to decompose biomass via
Supercritical Water Gasification of biomass. This type of
biomass gasification can be used to produce hydrocarbon fuels for use in an efficient combustion device or to produce hydrogen for use in a fuel cell. In the latter case, hydrogen yield can be much higher than the hydrogen content of the biomass due to steam reforming where water is a hydrogen-providing participant in the overall reaction.
Dry-cleaning
Supercritical carbon dioxide (SCD) can be used instead of PERC (
perchloroethylene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2 . It is a colorless li ...
) or other undesirable solvents for
dry-cleaning
Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water.
Dry cleaning still involves liquid, but clothes are instead soaked in a water-free liquid solvent. Tetrachloroethylene (perchloroethylene), known in ...
. Supercritical carbon dioxide sometimes
intercalates into buttons, and, when the SCD is depressurized, the buttons pop, or break apart. Detergents that are soluble in carbon dioxide improve the solvating power of the solvent. CO
2-based dry cleaning equipment uses liquid CO
2, not supercritical CO
2, to avoid damage to the buttons.
Supercritical fluid chromatography
Supercritical fluid chromatography
Supercritical fluid chromatography (SFC) is a form of normal phase chromatography that uses a supercritical fluid such as carbon dioxide as the mobile phase. It is used for the analysis and purification of low to moderate molecular weight, thermal ...
(SFC) can be used on an analytical scale, where it combines many of the advantages of
high performance liquid chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
(HPLC) and
gas chromatography (GC). It can be used with non-volatile and thermally labile analytes (unlike GC) and can be used with the universal
flame ionization detector
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ion per unit time make this a mass sensitive instrument. Standalo ...
(unlike HPLC), as well as producing narrower peaks due to rapid diffusion. In practice, the advantages offered by SFC have not been sufficient to displace the widely used HPLC and GC, except in a few cases such as
chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
separations and analysis of high-molecular-weight hydrocarbons. For manufacturing, efficient preparative
simulated moving bed In manufacturing, the simulated moving bed (SMB) process is a highly engineered process for implementing chromatographic separation. It is used to separate one chemical compound or one class of chemical compounds from one or more other chemical com ...
units are available. The purity of the final products is very high, but the cost makes it suitable only for very high-value materials such as pharmaceuticals.
Chemical reactions
Changing the conditions of the reaction solvent can allow separation of phases for product removal, or single phase for reaction. Rapid diffusion accelerates diffusion controlled reactions. Temperature and pressure can tune the reaction down preferred pathways, e.g., to improve yield of a particular
chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
. There are also significant environmental benefits over conventional organic solvents. Industrial syntheses that are performed at supercritical conditions include those of
polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bo ...
from supercritical
ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
,
isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group ( chemical formula ) it is the s ...
from supercritical
propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petro ...
,
2-butanol from supercritical
butene
Butene, also known as butylene, is an alkene with the formula . The word ''butene'' may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for ...
, and
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
from a supercritical mix of
nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
.
Other reactions were, in the past, performed industrially in supercritical conditions, including the synthesis of
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
and thermal (non-catalytic) oil cracking. Because of the development of effective
catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, the required temperatures of those two processes have been reduced and are no longer supercritical.
Impregnation and dyeing
Impregnation is, in essence, the converse of extraction. A substance is dissolved in the supercritical fluid, the solution flowed past a solid substrate, and is deposited on or dissolves in the substrate. Dyeing, which is readily carried out on polymer fibres such as polyester using disperse (non-ionic)
dyes, is a special case of this. Carbon dioxide also dissolves in many polymers, considerably swelling and plasticising them and further accelerating the diffusion process.
Nano and micro particle formation
The formation of small particles of a substance with a narrow size distribution is an important process in the pharmaceutical and other industries. Supercritical fluids provide a number of ways of achieving this by rapidly exceeding the
saturation point of a solute by dilution, depressurization or a combination of these. These processes occur faster in supercritical fluids than in liquids, promoting
nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that deter ...
or
spinodal decomposition
Spinodal decomposition is a mechanism by which a single thermodynamic phase spontaneously separates into two phases (without nucleation). Decomposition occurs when there is no thermodynamic barrier to phase separation. As a result, phase separatio ...
over
crystal growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists of the a ...
and yielding very small and regularly sized particles. Recent supercritical fluids have shown the capability to reduce particles up to a range of 5-2000 nm.
Generation of pharmaceutical cocrystals
Supercritical fluids act as a new media for the generation of novel crystalline forms of APIs (Active Pharmaceutical Ingredients) named as pharmaceutical cocrystals. Supercritical fluid technology offers a new platform that allows a single-step generation of particles that are difficult or even impossible to obtain by traditional techniques. The generation of pure and dried new cocrystals (crystalline molecular complexes comprising the API and one or more conformers in the crystal lattice) can be achieved due to unique properties of SCFs by using different supercritical fluid properties: supercritical CO
2 solvent power, anti-solvent effect and its atomization enhancement.
Supercritical drying
Supercritical drying
Supercritical drying, also known as critical point drying, is a process to remove liquid in a precise and controlled way. It is useful in the production of microelectromechanical systems (MEMS), the drying of spices, the production of aerogel, t ...
is a method of removing solvent without surface tension effects. As a liquid dries, the surface tension drags on small structures within a solid, causing distortion and shrinkage. Under supercritical conditions there is no surface tension, and the supercritical fluid can be removed without distortion. Supercritical drying is used in the manufacturing process of
aerogels and drying of delicate materials such as archaeological samples and biological samples for
electron microscopy.
Supercritical water electrolysis
Electrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
in a supercritical state, reduces the overpotentials found in other electrolysers, thereby improving the electrical efficiency of the production of oxygen and hydrogen.
Increased temperature reduces thermodynamic barriers and increases kinetics. No bubbles of oxygen or hydrogen are formed on the electrodes, therefore no insulating layer is formed between catalyst and water, reducing the ohmic losses. The gas-like properties provide rapid mass transfer.
Supercritical water oxidation
Supercritical water oxidation uses supercritical water as a medium in which to oxidize hazardous waste, eliminating production of toxic combustion products that burning can produce.
The waste product to be oxidised is dissolved in the supercritical water along with molecular oxygen (or an oxidising agent that gives up oxygen upon decomposition, e.g.
hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
) at which point the oxidation reaction occurs.
Supercritical water hydrolysis
Supercritical hydrolysis is a method of converting all biomass polysaccharides as well the associated lignin into low molecular compounds by contacting with water alone under supercritical conditions. The supercritical water, acts as a solvent, a supplier of bond-breaking thermal energy, a heat transfer agent and as a source of hydrogen atoms. All polysaccharides are converted into simple sugars in near-quantitative yield in a second or less. The aliphatic inter-ring linkages of lignin are also readily cleaved into free radicals that are stabilized by hydrogen originating from the water. The aromatic rings of the lignin are unaffected under short reaction times so that the lignin-derived products are low molecular weight mixed phenols. To take advantage of the very short reaction times needed for cleavage a continuous reaction system must be devised. The amount of water heated to a supercritical state is thereby minimized.
Supercritical water gasification
Supercritical water gasification is a process of exploiting the beneficial effect of supercritical water to convert aqueous biomass streams into clean water and gases like H
2, CH
4, CO
2, CO etc.
Supercritical fluid in power generation
The
efficiency of a
heat engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state ...
is ultimately dependent on the temperature difference between heat source and sink (
Carnot cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynam ...
). To improve efficiency of
power stations
A power station, also referred to as a power plant and sometimes generating station or generating plant, is an industrial facility for the generation of electric power. Power stations are generally connected to an electrical grid.
Many po ...
the
operating temperature
An operating temperature is the allowable temperature range of the local ambient environment at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the de ...
must be raised. Using water as the working fluid, this takes it into supercritical conditions. Efficiencies can be raised from about 39% for subcritical operation to about 45% using current technology.
Supercritical water reactors (SCWRs) are promising advanced nuclear systems that offer similar thermal efficiency gains. Carbon dioxide can also be used in supercritical cycle nuclear power plants, with similar efficiency gains. Many coal-fired
supercritical steam generator
A supercritical steam generator is a type of boiler that operates at supercritical pressure, frequently used in the production of electric power.
In contrast to a subcritical boiler in which bubbles can form, a supercritical steam generator op ...
s are operational all over the world, and have enhanced the efficiency of traditional steam-power plants.
Supercritical carbon dioxide is also proposed as a working fluid, which would have the advantage of lower critical pressure than water, but issues with corrosion are not yet fully solved. One proposed application is the
Allam cycle. Both carbon dioxide and water are
neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely mo ...
s, but they have a lower density as supercritical fluids than liquid water does. This allows nuclear reactors with those supercritical fluids as a primary coolant to run in a reduced moderation mode ("semi-fast" or "epithermal") but not usually as a
fast neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
reactor. On the other hand, some extra moderation would have to be provided for a fully thermal neutron spectrum.
Biodiesel production
Conversion of vegetable oil to
biodiesel
Biodiesel is a form of diesel fuel derived from plants or animals and consisting of long-chain fatty acid esters. It is typically made by chemically reacting lipids such as animal fat (tallow), soybean oil, or some other vegetable oil with ...
is via a
transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can ...
reaction, where the
triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and ''glyceride'').
Triglycerides are the main constituents of body fat in humans and other vertebrates, as w ...
is converted to the methyl ester plus
glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
. This is usually done using
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
and
caustic
Caustic most commonly refers to:
* Causticity, a property of various corrosive substances
** Sodium hydroxide, sometimes called ''caustic soda''
** Potassium hydroxide, sometimes called ''caustic potash''
** Calcium oxide, sometimes called ''caus ...
or acid catalysts, but can be achieved using supercritical methanol without a catalyst. The method of using supercritical methanol for biodiesel production was first studied by Saka and his coworkers. This has the advantage of allowing a greater range and water content of feedstocks (in particular, used cooking oil), the product does not need to be washed to remove catalyst, and is easier to design as a continuous process.
Enhanced oil recovery and carbon capture and storage
Supercritical carbon dioxide is used to
enhance oil recovery in mature oil fields. At the same time, there is the possibility of using "
clean coal technology
Coal pollution mitigation, sometimes called clean coal, is a series of systems and technologies that seek to mitigate the health and environmental impact of coal; in particular air pollution from coal-fired power stations, and from coal burnt b ...
" to combine enhanced recovery methods with
carbon sequestration
Carbon sequestration is the process of storing carbon in a carbon pool. Carbon dioxide () is naturally captured from the atmosphere through biological, chemical, and physical processes. These changes can be accelerated through changes in land ...
. The CO
2 is separated from other
flue gases
Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced ...
, compressed to the supercritical state, and injected into geological storage, possibly into existing oil fields to improve yields.
At present, only schemes isolating fossil CO
2 from natural gas actually use carbon storage, (e.g.,
Sleipner gas field
Oil from the Sleipner field.
The Sleipner gas field is a natural gas field in the block 15/9 of the North Sea, about west of Stavanger, Norway. Two parts of the field are in production, Sleipner West (proven in 1974), and Sleipner East (1981) ...
), but there are many plans for future CCS schemes involving pre- or post- combustion CO
2. There is also the possibility to reduce the amount of CO
2 in the atmosphere by using
biomass
Biomass is plant-based material used as a fuel for heat or electricity production. It can be in the form of wood, wood residues, energy crops, agricultural residues, and waste from industry, farms, and households. Some people use the terms bi ...
to generate power and sequestering the CO
2 produced.
Enhanced geothermal system
The use of supercritical carbon dioxide, instead of water, has been examined as a geothermal working fluid.
Refrigeration
Supercritical carbon dioxide is also emerging as a useful high-temperature
refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
, being used in new,
CFC/
HFC-free domestic
heat pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing h ...
s making use of the
transcritical cycle
A transcritical cycle is a closed thermodynamic cycle where the working fluid goes through both subcritical and supercritical states. In particular, for power cycles the working fluid is kept in the liquid region during the compression phase ...
. These systems are undergoing continuous development with supercritical carbon dioxide heat pumps already being successfully marketed in Asia. The
EcoCute
The EcoCute is an energy-efficient electric heat pump, water heating and supply system that uses heat extracted from the air to heat water for domestic, industrial and commercial use. Instead of the more conventional ammonia or haloalkane gases, ...
systems from Japan are some of the first commercially successful high-temperature domestic water heat pumps.
Supercritical fluid deposition
Supercritical fluids can be used to deposit functional nanostructured films and nanometer-size particles of metals onto surfaces. The high diffusivities and concentrations of precursor in the fluid as compared to the vacuum systems used in
chemical vapour deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (substra ...
allow deposition to occur in a surface reaction rate limited regime, providing stable and uniform interfacial growth. This is crucial in developing more powerful electronic components, and metal particles deposited in this way are also powerful catalysts for chemical synthesis and electrochemical reactions. Additionally, due to the high rates of precursor transport in solution, it is possible to coat high surface area particles which under
chemical vapour deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (substra ...
would exhibit depletion near the outlet of the system and also be likely to result in unstable interfacial growth features such as
dendrites
Dendrites (from Greek δένδρον ''déndron'', "tree"), also dendrons, are branched protoplasmic extensions of a nerve cell that propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the n ...
. The result is very thin and uniform films deposited at rates much faster than
atomic layer deposition, the best other tool for particle coating at this size scale.
Antimicrobial properties
CO
2 at high pressures has
antimicrobial
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals ar ...
properties. While its effectiveness has been shown for various applications, the mechanisms of inactivation have not been fully understood although they have been investigated for more than 60 years.
History
In 1822, Baron
Charles Cagniard de la Tour
Baron Charles Cagniard de la Tour (31 March 1777 – 5 July 1859) was a French engineer and physicist. Charles Cagniard was born in Paris, and after attending the École Polytechnique became one of the ''ingénieurs géographiques''. He examined t ...
discovered the critical point of a substance in his famous
cannon
A cannon is a large- caliber gun classified as a type of artillery, which usually launches a projectile using explosive chemical propellant. Gunpowder ("black powder") was the primary propellant before the invention of smokeless powder ...
barrel experiments. Listening to discontinuities in the sound of a rolling
flint
Flint, occasionally flintstone, is a sedimentary cryptocrystalline form of the mineral quartz, categorized as the variety of chert that occurs in chalk or marly limestone. Flint was widely used historically to make stone tools and start fir ...
ball in a sealed cannon filled with fluids at various temperatures, he observed the critical temperature. Above this temperature, the densities of the liquid and gas
phases become equal and the distinction between them disappears, resulting in a single supercritical fluid phase.
See also
*
Supercritical adsorption
*
Transcritical cycle
A transcritical cycle is a closed thermodynamic cycle where the working fluid goes through both subcritical and supercritical states. In particular, for power cycles the working fluid is kept in the liquid region during the compression phase ...
*
Critical point (thermodynamics)
*
Iceland Deep Drilling Project
The Iceland Deep Drilling Project (IDDP) is a geothermal project established in 2000 by a consortium of the National Energy Authority of Iceland (Orkustofnun/OS) and four of Iceland's leading energy companies: Hitaveita Sudurnesja (HS), Landsvir ...
References
Further reading
*
External links
Handy calculatorfor density, enthalpy, entropy and other thermodynamic data of supercritical / water and others
videos to present supercritical fluid critical point and solubility in supercritical fluid
NewScientist Environment FOUND:The hottest water on Earth*
{{Authority control
Critical phenomena
Phases of matter
Gases
 Figures 1 and 2 show two-dimensional projections of a
Figures 1 and 2 show two-dimensional projections of a  Hydrothermal circulation occurs within the Earth's crust wherever fluid becomes heated and begins to convect. These fluids are thought to reach supercritical conditions under a number of different settings, such as in the formation of porphyry copper deposits or high temperature circulation of seawater in the sea floor. At mid-ocean ridges, this circulation is most evident by the appearance of hydrothermal vents known as "black smokers". These are large (metres high) chimneys of sulfide and sulfate minerals which vent fluids up to 400 °C. The fluids appear like great black billowing clouds of smoke due to the precipitation of dissolved metals in the fluid. It is likely that at depth many of these vent sites reach supercritical conditions, but most cool sufficiently by the time they reach the sea floor to be subcritical. One particular vent site, Turtle Pits, has displayed a brief period of supercriticality at the vent site. A further site, Beebe, in the Cayman Trough, is thought to display sustained supercriticality at the vent orifice.
Hydrothermal circulation occurs within the Earth's crust wherever fluid becomes heated and begins to convect. These fluids are thought to reach supercritical conditions under a number of different settings, such as in the formation of porphyry copper deposits or high temperature circulation of seawater in the sea floor. At mid-ocean ridges, this circulation is most evident by the appearance of hydrothermal vents known as "black smokers". These are large (metres high) chimneys of sulfide and sulfate minerals which vent fluids up to 400 °C. The fluids appear like great black billowing clouds of smoke due to the precipitation of dissolved metals in the fluid. It is likely that at depth many of these vent sites reach supercritical conditions, but most cool sufficiently by the time they reach the sea floor to be subcritical. One particular vent site, Turtle Pits, has displayed a brief period of supercriticality at the vent site. A further site, Beebe, in the Cayman Trough, is thought to display sustained supercriticality at the vent orifice.