|
Peng–Robinson Equation Of State
Cubic equations of state are a specific class of thermodynamic models for modeling the pressure of a gas as a function of temperature and density and which can be rewritten as a cubic function of the molar volume. Equations of state are generally applied in the fields of physical chemistry and chemical engineering, particularly in the modeling of vapor–liquid equilibrium and chemical engineering process design. Van der Waals equation of state The van der Waals equation of state may be written as : \left(p + \frac\right)\left(V_\text - b\right) = RT where T is the absolute temperature, p is the pressure, V_\text is the molar volume and R is the universal gas constant. Note that V_\text = V / n, where V is the volume, and n=N/N_\text, where n is the number of moles, N is the number of particles, and N_\text is the Avogadro constant. These definitions apply to all equations of state below as well. The substance-specific constants a and b can be calculated from the critical pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equation Of State
In physics, chemistry, and thermodynamics, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate for weakly polar gases at low pressures and moderate temperatures. This equation becomes increasingly inaccurate at higher pressures and lower temperatures, and fails to pred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
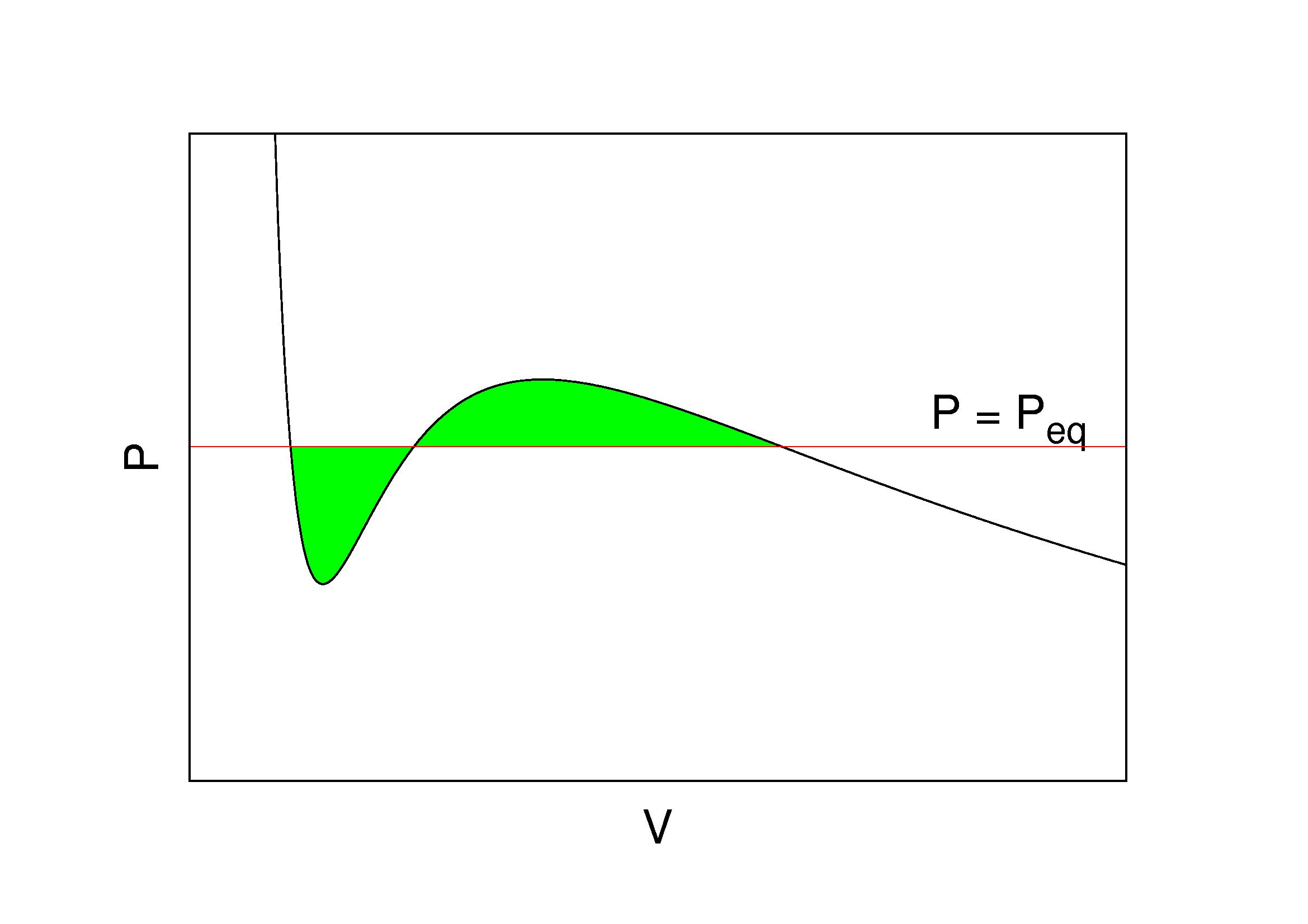
Maxwell Construction
In thermodynamic equilibrium, a necessary condition for stability is that pressure P does not increase with volume V. This basic consistency requirement—and similar ones for other ''conjugate'' pairs of variables—are sometimes violated in analytic models for first order phase transitions. The most famous case is the Van der Waals equation for real gases, see Fig. 1 where a typical ''isotherm'' is drawn (black curve). The Maxwell construction is a way of correcting this deficiency. The decreasing right hand part of the curve in Fig. 1 describes a diluted gas, while its left part describes a liquid. The intermediate (rising) part of the curve in Fig. 1 would be correct, if these two parts were to be joined smoothly—meaning in particular that the system would remain also in this region spatially uniform with a well defined density. But this is not what happens. If the volume of a vessel containing a fixed amount of liquid is expanded at constant temperature, there comes a point w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Departure Function
In thermodynamics, a departure function is defined for any thermodynamic property as the difference between the property as computed for an ideal gas and the property of the species as it exists in the real world, for a specified temperature ''T'' and pressure ''P''. Common departure functions include those for enthalpy, entropy, and internal energy. Departure functions are used to calculate real fluid extensive properties (i.e. properties which are computed as a difference between two states). A departure function gives the difference between the real state, at a finite volume or non-zero pressure and temperature, and the ideal state, usually at zero pressure or infinite volume and temperature. For example, to evaluate enthalpy change between two points ''h''(''v''1,''T''1) and ''h''(''v''2,''T''2) we first compute the enthalpy departure function between volume ''v''1 and infinite volume at ''T'' = ''T''1, then add to that the ideal gas enthalpy change due to the temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ding Yu Peng
Ding-Yu Peng is a professor of Chemical Engineering at the University of Saskatchewan. Under the direction of Donald B. Robinson, Peng introduced a two-parameter cubic equation of state now known as the Peng–Robinson equation of state during the 1970s while a research engineer. Early life Ding-Yu Peng was born in China sometime in 1943. Education Peng completed a degree in chemical engineering at the National Taiwan University in 1966. He studied for one year at Syracuse University during 1968-1969. Subsequently, he followed Leonard I. Stiel to the University of Missouri and obtained his PhD. in chemical engineering in 1973. Work under Robinson In late 1974, while working as a post-doctoral fellow under Robinson, the Natural Gas Processors Association requested a better gas model than was available at that time. Work on the Peng–Robinson equation of state was completed in 1975, and the results were published the following year. Teaching Peng taught thermodynami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Alberta
The University of Alberta, also known as U of A or UAlberta, is a Public university, public research university located in Edmonton, Alberta, Canada. It was founded in 1908 by Alexander Cameron Rutherford,"A Gentleman of Strathcona – Alexander Cameron Rutherford", Douglas R. Babcock, 1989, The University of Calgary Press, 2500 University Drive NW, Calgary, Alberta, Canada, the first premier of Alberta, and Henry Marshall Tory,"Henry Marshall Tory, A Biography", originally published 1954, current edition January 1992, E.A. Corbett, Toronto: Ryerson Press, the university's first president. It was enabled through the Post-secondary Learning Act''.'' The university is considered a "comprehensive academic and research university" (CARU), which means that it offers a range of academic and professional programs that generally lead to undergraduate and graduate level credentials. The university comprises four campuses in Edmonton, an Augustana Campus in Camrose, Alberta, Camrose, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acentric Factor
The acentric factor is a conceptual number introduced by Kenneth Pitzer in 1955, proven to be useful in the description of fluids. It has become a standard for the phase characterization of single & pure components, along with other state description parameters such as molecular weight, critical temperature, critical pressure, and critical volume (or critical compressibility). Pitzer defined from the relationship :\omega = - \log_ (p^_r) - 1, T_r = 0.7 where p^_r = \frac is the reduced saturation vapor pressure and T_r = \frac is the reduced temperature. The acentric factor is said to be a measure of the non-sphericity (centricity) of molecules. As it increases, the vapor curve is "pulled" down, resulting in higher boiling points. For many monatomic fluids, p_r^ T_r = 0.7 is close to 0.1, which leads to \omega \to 0. In many cases, T_r = 0.7 lies above the boiling temperature of liquids at atmosphere pressure. Values of can be determined for any fluid from accurate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy, food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Equation
In chemistry and thermodynamics, the Van der Waals equation (or Van der Waals equation of state) is an equation of state which extends the ideal gas law to include the effects of interaction between molecules of a gas, as well as accounting for the finite size of the molecules. The ideal gas law treats gas molecules as point particles that interact with their containers but not each other, meaning they neither take up space nor change kinetic energy during collisions (i.e. all collisions are perfectly elastic). The ideal gas law states that the volume ''V'' occupied by ''n'' moles of any gas has a pressure ''P'' at temperature ''T'' given by the following relationship, where ''R'' is the gas constant: :PV=nRT To account for the volume occupied by real gas molecules, the Van der Waals equation replaces V/n in the ideal gas law with (V_m-b), where ''Vm'' is the molar volume of the gas and ''b'' is the volume occupied by the molecules of one mole: :P(V_m - b)=R T The secon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theorem Of Corresponding States
According to van der Waals, the theorem of corresponding states (or principle/law of corresponding states) indicates that all fluids, when compared at the same reduced temperature and reduced pressure, have approximately the same compressibility factor and all deviate from ideal gas behavior to about the same degree. Material constants that vary for each type of material are eliminated, in a recast reduced form of a constitutive equation. The reduced variables are defined in terms of critical variables. The principle originated with the work of Johannes Diderik van der Waals in about 1873 by Walter M. Kalback and Kenneth E. Starling, Chemical Engineering Department, University of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |