Sparkling Water on:
[Wikipedia]
[Google]
[Amazon]
Carbonated water (also known as soda water, sparkling water, fizzy water, club soda, water with gas, in many places as mineral water, or especially in the United States as seltzer or seltzer water) is  Club soda and sparkling mineral water and some other sparkling waters contain added or dissolved
Club soda and sparkling mineral water and some other sparkling waters contain added or dissolved
 Many
Many 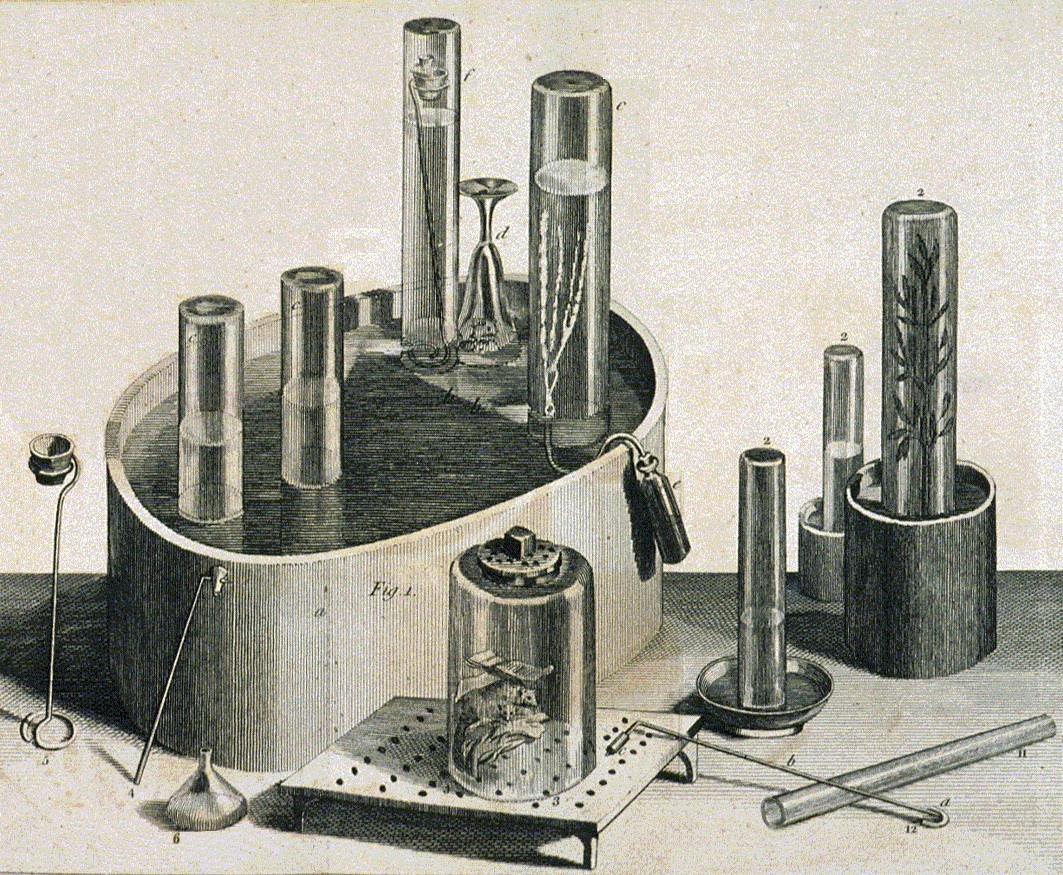 In 1767 Priestley discovered a method of infusing water with carbon dioxide by pouring water back and forth above a beer vat at a local brewery in
In 1767 Priestley discovered a method of infusing water with carbon dioxide by pouring water back and forth above a beer vat at a local brewery in
New York Times. Retrieved 10 January 2015 The air blanketing the fermenting beer—called 'fixed air'—was known to kill mice suspended in it. Priestley found water thus treated had a pleasant taste, and he offered it to friends as a cool, refreshing drink. In 1772, Priestley published a paper titled ''Impregnating Water with Fixed Air'' in which he describes dripping "oil of vitriol" (
 In the United States, plain carbonated water was generally known either as ''soda water'', due to the sodium salts it contained, or ''seltzer water'', deriving from the German town
In the United States, plain carbonated water was generally known either as ''soda water'', due to the sodium salts it contained, or ''seltzer water'', deriving from the German town
 The
The
 The
The
 In 1872, soft drink maker
In 1872, soft drink maker
 Soda makers or soda carbonators, known as ''countertop carborators'', are appliances that carbonate water with multiple-use carbon dioxide canisters. Soda makers may reach a higher level of carbonation than home soda siphons. A variety of systems are produced by manufacturers and hobbyists. The commercial units may be sold with concentrated syrup for making flavored soft drinks.
One major producer of soda carbonators is SodaStream. Their products were popular during the 1970s and 1980s in the United Kingdom, and are associated with nostalgia for that period and have experienced a comeback in the 2000s.
Soda makers or soda carbonators, known as ''countertop carborators'', are appliances that carbonate water with multiple-use carbon dioxide canisters. Soda makers may reach a higher level of carbonation than home soda siphons. A variety of systems are produced by manufacturers and hobbyists. The commercial units may be sold with concentrated syrup for making flavored soft drinks.
One major producer of soda carbonators is SodaStream. Their products were popular during the 1970s and 1980s in the United Kingdom, and are associated with nostalgia for that period and have experienced a comeback in the 2000s.
 The process of dissolving carbon dioxide in water is called
The process of dissolving carbon dioxide in water is called
/ref> The gas dissolves in the water, and a top-off fill of carbon dioxide is added to pressurize the siphon to approximately , some higher than is present in fermenting
The Priestley Society
Priestley's paper ''Impregnating Water with Fixed Air'' 1772
{{DEFAULTSORT:Carbonated Water Carbonated drinks English inventions British culture Industrial gases Soft drinks 18th-century inventions Drinks
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
containing dissolved carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
gas, either artificially injected under pressure or occurring due to natural geological processes. Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
In inorganic ch ...
causes small bubbles to form, giving the water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
an effervescent
Effervescence is the escape of gas from an aqueous solution and the foaming or fizzing that results from that release. The word effervescence is derived from the Latin verb ''fervere'' (to boil), preceded by the adverb ''ex''. It has the same lin ...
quality. Common forms include sparkling natural mineral water
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. Mineral water may usually be still or sparkling (carbonated/effervescent) according to the presence or absence of added gases.
Tra ...
, club soda
Club soda is a manufactured form of carbonated water, commonly used as a drink mixer. Sodium bicarbonate, potassium sulfate, potassium bicarbonate, potassium citrate, or sodium citrate is artificially added to replicate constituents commonly fo ...
, and commercially-produced sparkling water. Club soda and sparkling mineral water and some other sparkling waters contain added or dissolved
Club soda and sparkling mineral water and some other sparkling waters contain added or dissolved mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ( ...
s such as potassium bicarbonate
Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.
Production and reactivity
It is manufactured by treating an ...
, sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
, sodium citrate Sodium citrate may refer to any of the sodium salts of citric acid (though most commonly the third):
* Monosodium citrate
* Disodium citrate
* Trisodium citrate
The three forms of salt are collectively known by the E number E331.
Applications
F ...
, or potassium sulfate
Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, pro ...
. These occur naturally in some mineral waters but are also commonly added artificially to manufactured waters to mimic a natural flavor profile and offset the acidity of introducing carbon dioxide gas. Various carbonated waters are sold in bottles and cans, with some also produced on demand by commercial carbonation systems in bars and restaurants, or made at home using a carbon dioxide cartridge.
It is thought that the first person to aerate water with carbon dioxide was William Brownrigg
William Brownrigg ( – 6 January 1800) was a British doctor and scientist, who practised at Whitehaven in Cumberland. While there, Brownrigg carried out experiments that earned him the Copley Medal in 1766 for his work on carbonic acid gas. He ...
in 1740. Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
invented carbonated water, independently and by accident, in 1767 when he discovered a method of infusing water with carbon dioxide after having suspended a bowl of water above a beer vat at a brewery in Leeds
Leeds () is a city and the administrative centre of the City of Leeds district in West Yorkshire, England. It is built around the River Aire and is in the eastern foothills of the Pennines. It is also the third-largest settlement (by populati ...
, England. He wrote of the "peculiar satisfaction" he found in drinking it, and in 1772 he published a paper entitled ''Impregnating Water with Fixed Air''. Priestley's apparatus, almost identical to that used by Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
five years earlier, which featured a bladder between the generator and the absorption tank to regulate the flow of carbon dioxide, was soon joined by a wide range of others. However, it was not until 1781 that carbonated water began being produced on a large scale with the establishment of companies specialized in producing artificial mineral water. The first factory was built by Thomas Henry of Manchester
Manchester () is a city in Greater Manchester, England. It had a population of 552,000 in 2021. It is bordered by the Cheshire Plain to the south, the Pennines to the north and east, and the neighbouring city of Salford to the west. The t ...
, England. Henry replaced the bladder in Priestley's system with large bellows.
While Priestley's discovery ultimately led to the creation of the soft drink
A soft drink (see § Terminology for other names) is a drink that usually contains water (often carbonated), a sweetener, and a natural and/or artificial flavoring. The sweetener may be a sugar, high-fructose corn syrup, fruit juice, a su ...
industry—which began in 1783 when Johann Jacob Schweppe
Johann Jacob Schweppe (, ) (16 March 1740 – 18 November 1821) was a German-Swiss watchmaker and amateur scientist who developed the first practical process to manufacture bottled carbonated mineral water, based on a process discovered by Joseph ...
founded Schweppes
Schweppes (, ) is a beverage brand that originated in the Republic of Geneva; it is made, bottled and distributed worldwide by multiple international conglomerates, depending on licensing and region, that manufacture and sell soft drinks. Schwep ...
to sell bottled soda water, he did not benefit financially from his invention. Priestley did however receive scientific recognition when the Council of the Royal Society
The Royal Society, formally The Royal Society of London for Improving Natural Knowledge, is a learned society and the United Kingdom's national academy of sciences. The society fulfils a number of roles: promoting science and its benefits, re ...
"were moved to reward its discoverer with the Copley Medal
The Copley Medal is an award given by the Royal Society, for "outstanding achievements in research in any branch of science". It alternates between the physical sciences or mathematics and the biological sciences. Given every year, the medal is t ...
" in 1772.
Composition
Natural and manufactured carbonated waters may contain a small amount ofsodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, sodium citrate Sodium citrate may refer to any of the sodium salts of citric acid (though most commonly the third):
* Monosodium citrate
* Disodium citrate
* Trisodium citrate
The three forms of salt are collectively known by the E number E331.
Applications
F ...
, sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
, potassium bicarbonate
Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.
Production and reactivity
It is manufactured by treating an ...
, potassium citrate
Potassium citrate (also known as tripotassium citrate) is a potassium salt of citric acid with the molecular formula K3C6H5O7. It is a white, hygroscopic crystalline powder. It is odorless with a saline taste. It contains 38.28% potassium by mass ...
, potassium sulfate
Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, pro ...
, or disodium phosphate
Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, ...
, depending on the product. These occur naturally in mineral water
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. Mineral water may usually be still or sparkling (carbonated/effervescent) according to the presence or absence of added gases.
Tra ...
s but are added artificially to commercially produced waters to mimic a natural flavor profile and offset the acidity of introducing carbon dioxide gas (which creates low 5-6 pH carbonic acid solution when dissolved in water).
Artesian wells in such places as Mihalkovo in the Bulgarian Rhodope Mountains
The Rhodopes (; bg, Родопи, ; el, Ροδόπη, ''Rodopi''; tr, Rodoplar) are a mountain range in Southeastern Europe, and the largest by area in Bulgaria, with over 83% of its area in the southern part of the country and the remainder in ...
, Medžitlija in North Macedonia
North Macedonia, ; sq, Maqedonia e Veriut, (Macedonia before February 2019), officially the Republic of North Macedonia,, is a country in Southeast Europe. It gained independence in 1991 as one of the successor states of Socialist Feder ...
, and most notably in Selters
Selters is a German brand of natural mineral water sourced from wells in the area of Selters in Hesse, at the Taunus mountains.
The water has been known since the Bronze Age and famous as a natural soda water because of its high concentration ...
in the German Taunus
The Taunus is a mountain range in Hesse, Germany, located north of Frankfurt. The tallest peak in the range is ''Großer Feldberg'' at 878 m; other notable peaks are ''Kleiner Feldberg'' (825 m) and ''Altkönig'' (798 m).
The Taunus range spans ...
mountains, produce naturally effervescent
Effervescence is the escape of gas from an aqueous solution and the foaming or fizzing that results from that release. The word effervescence is derived from the Latin verb ''fervere'' (to boil), preceded by the adverb ''ex''. It has the same lin ...
mineral waters.
Health effects
By itself, carbonated water appears to have little impact on health. Carbonated water such as club soda or sparkling water is defined in US law as a food ofminimal nutritional value In United States law, a food of minimal nutritional value is one that USDA has determined contain little to no nutritional value; these foods may not be sold in competition with the school lunch and breakfast programs. For example, sugar candy, soda ...
, even if minerals, vitamins
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an essential micronutrient that an organism needs in small quantities for the proper functioning of its metabolism. Essential nutrien ...
, or artificial sweeteners
A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie () or low-calorie sweetener. Artificial sweeteners may b ...
have been added to it.
Carbonated water does not appear to have an effect on gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) or gastro-oesophageal reflux disease (GORD) is one of the upper gastrointestinal chronic diseases where stomach content persistently and regularly flows up into the esophagus, resulting in symptoms and/ ...
. There is tentative evidence that carbonated water may help with constipation
Constipation is a bowel dysfunction that makes bowel movements infrequent or hard to pass. The stool is often hard and dry. Other symptoms may include abdominal pain, bloating, and feeling as if one has not completely passed the bowel movement ...
among people who have had a stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functionin ...
.
Acid erosion
While carbonated water is somewhat acidic, this acidity can be partially neutralized bysaliva
Saliva (commonly referred to as spit) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be ...
. A study found that sparkling mineral water is slightly more erosive to teeth than non-carbonated water but is about 1% as corrosive as soft drinks are. A 2017 study by the American Dental Association showed that it would take over 100 years of daily sparkling water consumption to cause damage to human teeth - a claim that could not apply if there is added sugar or artificial flavorings, which often include citric acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ...
and other fruit acids, predicted to have an impact on human teeth.
Chemistry
Carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
gas dissolved in water at a low concentration (0.2–1.0%) creates carbonic acid (H2CO3) according to the following reaction:
: (l) + (g) ⇌ (aq)
The acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
gives carbonated water a slightly tart flavor. The pH level between 5 and 6 is approximately in between apple juice
Apple juice is a fruit juice made by the maceration and pressing of an apple. The resulting expelled juice may be further treated by enzymatic and centrifugal clarification to remove the starch and pectin, which holds fine particulate in suspe ...
and orange juice
Orange juice is a liquid extract of the orange (fruit), orange tree fruit, produced by squeezing or reaming oranges. It comes in several different varieties, including blood orange, navel oranges, valencia orange, clementine, and tangerine. A ...
in acidity, but much less acidic than the acid in the stomach. A normal, healthy human body maintains pH equilibrium via acid–base homeostasis
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases (i.e. the pH) in the ECF is crucial for the normal physiology of the body—and for cellular ...
and will not be materially adversely affected by consumption of plain carbonated water. Alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a base (chemistry), basic, ionic compound, ionic salt (chemistry), salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as ...
salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively cha ...
, such as sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
, potassium bicarbonate
Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.
Production and reactivity
It is manufactured by treating an ...
, or potassium citrate
Potassium citrate (also known as tripotassium citrate) is a potassium salt of citric acid with the molecular formula K3C6H5O7. It is a white, hygroscopic crystalline powder. It is odorless with a saline taste. It contains 38.28% potassium by mass ...
, will increase pH.
The amount of a gas that can be dissolved in water is described by Henry's Law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulat ...
. In the carbonization process, water is chilled, optimally to just above freezing, to maximize the amount of carbon dioxide that can be dissolved in it. Higher gas pressure and lower temperature cause more gas to dissolve in the liquid. When the temperature is raised or the pressure is reduced (as happens when a container of carbonated water is opened), carbon dioxide effervesces, thereby escaping from the solution.
History
 Many
Many alcoholic drink
An alcoholic beverage (also called an alcoholic drink, adult beverage, or a drink) is a drink that contains ethanol, a type of alcohol that acts as a drug and is produced by fermentation of grains, fruits, or other sources of sugar. The c ...
s, such as beer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
, champagne
Champagne (, ) is a sparkling wine originated and produced in the Champagne wine region of France under the rules of the appellation, that demand specific vineyard practices, sourcing of grapes exclusively from designated places within it, spe ...
, cider
Cider ( ) is an alcoholic beverage made from the fermented juice of apples. Cider is widely available in the United Kingdom (particularly in the West Country) and the Republic of Ireland. The UK has the world's highest per capita consumption, ...
, and spritzer
A spritzer is a tall, chilled drink, usually made with white wine and carbonated water or sparkling mineral water. Fermented simple syrup can be used instead of white wine to keep it sweet but flavor neutral.
Origin
''Spritzer'' is derived fro ...
, were naturally carbonated through the fermentation process for centuries. In 1662 Christopher Merret
Christopher Merret FRSFRCP(16 February 1614/1615 – 19 August 1695), also spelt Merrett, was an English physician and scientist. He was the first to document the deliberate addition of sugar for the production of sparkling wine, and produced ...
created 'sparkling wine'. William Brownrigg
William Brownrigg ( – 6 January 1800) was a British doctor and scientist, who practised at Whitehaven in Cumberland. While there, Brownrigg carried out experiments that earned him the Copley Medal in 1766 for his work on carbonic acid gas. He ...
was apparently the first to produce artificial carbonated water, in the early 1740s, by using carbon dioxide taken from mines. In 1750 the Frenchman Gabriel François Venel
Gabriel François Venel (23 August 1723, Tourbes – 29 October 1775, Pézenas) was a French chemist, physician and a contributor to the ''Encyclopédie'', (673 items; In 1742 he obtained his doctorate in medicine from the University of Montpellie ...
also produced artificial carbonated water, though he misunderstood the nature of the gas that caused the carbonation. In 1764, Irish chemist Dr. Macbride infused water with carbon dioxide as part of a series of experiments on fermentation and putrefaction. In 1766 Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
devised an aerating apparatus that would inspire Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
to carry out his own experiments with regards to carbonated waters. Cavendish was also aware of Brownrigg's observations at this time and published a paper on his own experiments on a nearby source of mineral water at the beginning of January in the next year.
 In 1767 Priestley discovered a method of infusing water with carbon dioxide by pouring water back and forth above a beer vat at a local brewery in
In 1767 Priestley discovered a method of infusing water with carbon dioxide by pouring water back and forth above a beer vat at a local brewery in Leeds
Leeds () is a city and the administrative centre of the City of Leeds district in West Yorkshire, England. It is built around the River Aire and is in the eastern foothills of the Pennines. It is also the third-largest settlement (by populati ...
, England."The Man Who Discovered Oxygen and Gave the World Soda Water"New York Times. Retrieved 10 January 2015 The air blanketing the fermenting beer—called 'fixed air'—was known to kill mice suspended in it. Priestley found water thus treated had a pleasant taste, and he offered it to friends as a cool, refreshing drink. In 1772, Priestley published a paper titled ''Impregnating Water with Fixed Air'' in which he describes dripping "oil of vitriol" (
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
) onto chalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Chalk ...
to produce carbon dioxide gas, and encouraging the gas to dissolve into an agitated bowl of water. Priestley referred to his invention of this treated water as being his "happiest" discovery.
Priestley's apparatus, which was very similar to that invented by Henry Cavendish five years earlier, featured a bladder between the generator and the absorption tank to regulate the flow of carbon dioxide, and was soon joined by a wide range of others, but it was not until 1781 that carbonated water began being produced on a large scale with the establishment of companies specialized in producing artificial mineral water. The first factory was built by Thomas Henry of Manchester
Manchester () is a city in Greater Manchester, England. It had a population of 552,000 in 2021. It is bordered by the Cheshire Plain to the south, the Pennines to the north and east, and the neighbouring city of Salford to the west. The t ...
, England. Henry replaced the bladder in Priestley's system with large bellows. J. J. Schweppe developed a process to manufacture bottled carbonated mineral water based on the discovery of Priestley, founding the Schweppes
Schweppes (, ) is a beverage brand that originated in the Republic of Geneva; it is made, bottled and distributed worldwide by multiple international conglomerates, depending on licensing and region, that manufacture and sell soft drinks. Schwep ...
Company in Geneva in 1783. Schweppes regarded Priestley as “the father of our industry”. In 1792 he moved to London to develop the business there. In 1799 Augustine Thwaites founded Thwaites' Soda Water in Dublin. A ''London Globe'' article claims that this company was the first to patent and sell "Soda Water" under that name. The article says that in the hot summer of 1777 in London "aerated waters" (that is, carbonated) were selling well but there was as yet no mention of "soda water", though the first effervescent drinks were probably made using " soda powders" containing bicarbonate of soda
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
and tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally i ...
. The name soda water arose from the fact that soda (sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
or bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
) was often added to adjust the taste and pH.
Modern carbonated water is made by injecting pressurized carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
into water. The pressure increases the solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
and allows more carbon dioxide to dissolve than would be possible under standard atmospheric pressure
The standard atmosphere (symbol: atm) is a unit of pressure defined as Pa. It is sometimes used as a ''reference pressure'' or ''standard pressure''. It is approximately equal to Earth's average atmospheric pressure at sea level.
History
The s ...
. When the bottle is opened, the pressure is released, allowing gas to exit the solution, forming the characteristic bubbles.
Etymology
 In the United States, plain carbonated water was generally known either as ''soda water'', due to the sodium salts it contained, or ''seltzer water'', deriving from the German town
In the United States, plain carbonated water was generally known either as ''soda water'', due to the sodium salts it contained, or ''seltzer water'', deriving from the German town Selters
Selters is a German brand of natural mineral water sourced from wells in the area of Selters in Hesse, at the Taunus mountains.
The water has been known since the Bronze Age and famous as a natural soda water because of its high concentration ...
renowned for its mineral springs
Mineral springs are naturally occurring springs that produces hard water, water that contains dissolved minerals. Salts, sulfur compounds, and gases are among the substances that can be dissolved in the spring water during its passage underg ...
.
Sodium salts were added to plain water both as flavoring (to mimic famed mineral water
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. Mineral water may usually be still or sparkling (carbonated/effervescent) according to the presence or absence of added gases.
Tra ...
s, such as naturally effervescent ''Selters
Selters is a German brand of natural mineral water sourced from wells in the area of Selters in Hesse, at the Taunus mountains.
The water has been known since the Bronze Age and famous as a natural soda water because of its high concentration ...
'', ''Vichy water'' and ''Saratoga water'') and acidity regulators (to offset the acidic 5-6 pH carbonic acid created when carbon dioxide is dissolved in water).
In the 1950s the term club soda
Club soda is a manufactured form of carbonated water, commonly used as a drink mixer. Sodium bicarbonate, potassium sulfate, potassium bicarbonate, potassium citrate, or sodium citrate is artificially added to replicate constituents commonly fo ...
began to be popularized.
In the 1970s marketing-driven terms such as ''sparkling water'' gained favor, with an explosion of consumption of the naturally carbonated Perrier
Perrier ( , also , ) is a French brand of natural bottled mineral water obtained at its source in Vergèze, located in the Gard ''département''. Perrier is known for its carbonation and its distinctive green bottle.
Perrier was part of the ...
water .
Generally, ''seltzer water'' has no added sodium salts, while ''club soda'' still retains some sodium salts.
Products for carbonating water
Home
Soda siphons
 The
The soda siphon
The soda siphon (sometimes spelled syphon), also known as the seltzer bottle or siphon seltzer bottle, is a device for storing and dispensing carbonated beverages (typically carbonated water) while maintaining the internal pressure, thereby preve ...
, or seltzer bottle—a glass or metal pressure vessel with a release valve and spout for dispensing pressurized soda water—was a common sight in bars and in early- to mid-20th-century homes where it became a symbol of middle-class affluence.
The gas pressure in a siphon drives soda water up through a tube inside the siphon when a valve lever at the top is depressed. Commercial soda siphons came pre-charged with water and gas and were returned to the retailer for exchange when empty. A deposit scheme ensured they were not otherwise thrown away.
Home soda siphons can carbonate flatwater through the use of a small disposable steel bulb containing carbon dioxide. The bulb is pressed into the valve assembly at the top of the siphon, the gas injected, then the bulb withdrawn. Soda water made in this way tends not to be as carbonated as commercial soda water because water from the refrigerator is not chilled as much as possible, and the pressure of carbon dioxide is limited to that available from the cartridge rather than the high-pressure pumps in a commercial carbonation plant.
Gasogene
 The
The gasogene
The gasogene (or gazogene or seltzogene) is a late Victorian device for producing carbonated water. It consists of two linked glass globes: the lower contained water or other drink to be made sparkling, the upper a mixture of tartaric acid and ...
(or gazogene, or seltzogene) is a late Victorian device for producing carbonated water. It consists of two linked glass globes: the lower contained water or other drink to be made sparkling, the upper a mixture of tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally i ...
and sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
that reacts to produce carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
. The produced gas pushes the liquid in the lower container up a tube and out of the device. The globes are surrounded by a wicker
Wicker is the oldest furniture making method known to history, dating as far back as 5,000 years ago. It was first documented in ancient Egypt using pliable plant material, but in modern times it is made from any pliable, easily woven material. ...
or wire protective mesh, as they have a tendency to explode.
Codd-neck bottles
 In 1872, soft drink maker
In 1872, soft drink maker Hiram Codd
Hiram Codd (10 January 1838 – 18 February 1887) was an English engineer and inventor. In 1872, he patented a bottle filled under gas pressure which pushed a marble against a rubber washer in the neck, creating a seal for soft drinks. Th ...
of Camberwell
Camberwell () is a district of South London, England, in the London Borough of Southwark, southeast of Charing Cross.
Camberwell was first a village associated with the church of St Giles and a common of which Goose Green is a remnant. This e ...
, London, designed and patented the Codd-neck bottle
A Codd-neck bottle (more commonly known as a Codd bottle or a marble bottle) is a type of bottle used for carbonated drinks. It has a closing design based on a glass marble which is held against a rubber seal, which sits within a recess in the li ...
, designed specifically for carbonated
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
In inorganic ch ...
drinks. The ''Codd-neck bottle'' encloses a marble
Marble is a metamorphic rock composed of recrystallized carbonate minerals, most commonly calcite or Dolomite (mineral), dolomite. Marble is typically not Foliation (geology), foliated (layered), although there are exceptions. In geology, the ...
and a rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
washer/gasket in the neck. The bottles were filled upside down, and pressure of the gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
in the bottle forced the marble against the washer, sealing in the carbonation. The bottle was pinched into a special shape to provide a chamber into which the marble was pushed to open the bottle. This prevented the marble from blocking the neck as the drink was poured.
Soon after its introduction, the bottle became extremely popular with the soft drink and brewing
Brewing is the production of beer by steeping a starch source (commonly cereal grains, the most popular of which is barley) in water and #Fermenting, fermenting the resulting sweet liquid with Yeast#Beer, yeast. It may be done in a brewery ...
industries mainly in the UK and the rest of Europe
Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...
, Asia
Asia (, ) is one of the world's most notable geographical regions, which is either considered a continent in its own right or a subcontinent of Eurasia, which shares the continental landmass of Afro-Eurasia with Africa. Asia covers an area ...
, and Australasia
Australasia is a region that comprises Australia, New Zealand and some neighbouring islands in the Pacific Ocean. The term is used in a number of different contexts, including geopolitically, physiogeographically, philologically, and ecologica ...
, though some alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
drinkers disdained the use of the bottle. R. White's, the biggest soft drinks company in London and south-east England when the bottle was introduced, was among the companies that sold their drinks in Codd's glass bottles. One etymology
Etymology ()The New Oxford Dictionary of English (1998) – p. 633 "Etymology /ˌɛtɪˈmɒlədʒi/ the study of the class in words and the way their meanings have changed throughout time". is the study of the history of the Phonological chan ...
of the term '' codswallop'' originates from beer sold in Codd bottles, though this is generally dismissed as a folk etymology
Folk etymology (also known as popular etymology, analogical reformation, reanalysis, morphological reanalysis or etymological reinterpretation) is a change in a word or phrase resulting from the replacement of an unfamiliar form by a more famili ...
.
The bottles were produced for many decades, but gradually declined in usage. Since children smashed the bottles to retrieve the marbles, vintage bottles are relatively rare and have become collector items, particularly in the UK. Due to the risk of explosion and injuries from fragmented glass pieces, use of this type of bottle is discouraged in most countries. The Codd-neck design is still used for the Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
ese soft drink Ramune
() is a Japanese carbonated soft drink. It was introduced in 1884 in Kobe by the British pharmacist Alexander Cameron Sim. Like Banta, an Indian lemon drink, is available in a Codd-neck bottle, a heavy glass bottle whose mouth is sealed by ...
and in the India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the so ...
n drink called Banta
Banta Soda, or Banta, also Goli Soda or Goti Soda and Fotash Jawl, is a popular carbonated lemon or orange-flavoured soft drink sold in India since the late 19th century in a distinctly shaped iconic Codd-neck bottle. The pressure created by ...
.
Soda makers
 Soda makers or soda carbonators, known as ''countertop carborators'', are appliances that carbonate water with multiple-use carbon dioxide canisters. Soda makers may reach a higher level of carbonation than home soda siphons. A variety of systems are produced by manufacturers and hobbyists. The commercial units may be sold with concentrated syrup for making flavored soft drinks.
One major producer of soda carbonators is SodaStream. Their products were popular during the 1970s and 1980s in the United Kingdom, and are associated with nostalgia for that period and have experienced a comeback in the 2000s.
Soda makers or soda carbonators, known as ''countertop carborators'', are appliances that carbonate water with multiple-use carbon dioxide canisters. Soda makers may reach a higher level of carbonation than home soda siphons. A variety of systems are produced by manufacturers and hobbyists. The commercial units may be sold with concentrated syrup for making flavored soft drinks.
One major producer of soda carbonators is SodaStream. Their products were popular during the 1970s and 1980s in the United Kingdom, and are associated with nostalgia for that period and have experienced a comeback in the 2000s.
Commercial
 The process of dissolving carbon dioxide in water is called
The process of dissolving carbon dioxide in water is called carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
In inorganic ch ...
. Commercial soda water in siphons is made by chilling filtered plain water to or below, optionally adding a sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
or potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
based alkaline compound such as sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
to neutralize the acid created when pressurizing the water with carbon dioxide (which creates high 8-10 pH carbonic acid-bicarbonate buffer solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
when dissolved in water).Carbonic acid, ''britannica.com''/ref> The gas dissolves in the water, and a top-off fill of carbon dioxide is added to pressurize the siphon to approximately , some higher than is present in fermenting
champagne
Champagne (, ) is a sparkling wine originated and produced in the Champagne wine region of France under the rules of the appellation, that demand specific vineyard practices, sourcing of grapes exclusively from designated places within it, spe ...
bottles.
In many modern restaurants and bars soda water is manufactured on-site using devices known as carbonators. Carbonators use mechanical pumps to pump water into a pressurized chamber where it is combined with carbon dioxide from pressurized tanks at approximately . The pressurized carbonated water then flows either directly to taps or mixing heads where flavoring is added before dispensing.
Uses
Carbonated beverages
Carbonated water is a key ingredient insoft drink
A soft drink (see § Terminology for other names) is a drink that usually contains water (often carbonated), a sweetener, and a natural and/or artificial flavoring. The sweetener may be a sugar, high-fructose corn syrup, fruit juice, a su ...
s, beverages that typically consist of carbonated water, a sweetener, and a flavoring such as cola
Cola is a carbonated soft drink flavored with vanilla, cinnamon, citrus oils and other flavorings. Cola became popular worldwide after the American pharmacist John Stith Pemberton invented Coca-Cola, a trademarked brand, in 1886, which was imita ...
, ginger
Ginger (''Zingiber officinale'') is a flowering plant whose rhizome, ginger root or ginger, is widely used as a spice
A spice is a seed, fruit, root, bark, or other plant substance primarily used for flavoring or coloring food. Spices ...
, or citrus
''Citrus'' is a genus of flowering plant, flowering trees and shrubs in the rue family, Rutaceae. Plants in the genus produce citrus fruits, including important crops such as Orange (fruit), oranges, Lemon, lemons, grapefruits, pomelos, and lim ...
.
Plain carbonated water or sparkling mineral water
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. Mineral water may usually be still or sparkling (carbonated/effervescent) according to the presence or absence of added gases.
Tra ...
is often consumed as an alternative to soft drinks. Club soda
Club soda is a manufactured form of carbonated water, commonly used as a drink mixer. Sodium bicarbonate, potassium sulfate, potassium bicarbonate, potassium citrate, or sodium citrate is artificially added to replicate constituents commonly fo ...
is carbonated water to which compounds such as sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
or potassium sulfate
Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, pro ...
have been added. Many manufacturers produce unsweetened sparkling water products that are lightly flavored by the addition of aromatic ingredients such as essential oils
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the o ...
. Carbonated water is often mixed with fruit juice to make sparkling alcoholic and non-alcoholic punches.
Alcoholic beverages
Carbonated water is adiluent
A diluent (also referred to as a filler, dilutant or thinner) is a Concentration, diluting agent. Certain fluids are too Viscosity, viscous to be pumped easily or too density, dense to flow from one particular point to the other. This can be prob ...
mixed with alcoholic beverages
An alcoholic beverage (also called an alcoholic drink, adult beverage, or a drink) is a drink that contains ethanol, a type of alcohol that acts as a drug and is produced by fermentation of grains, fruits, or other sources of sugar. The cons ...
where it is used to top-off the drink and provides a degree of 'fizz'.
Adding soda water to 'short' drinks such as spirits dilutes them and makes them 'long' not to be confused with long drinks such as those made with vermouth
Vermouth (, ) is an aromatized fortified wine, flavoured with various botanicals (roots, barks, flowers, seeds, herbs, and spices) and sometimes colored. The modern versions of the beverage were first produced in the mid- to late 18th centur ...
. Carbonated water also works well in short drinks made with whiskey
Whisky or whiskey is a type of distilled alcoholic beverage made from fermented grain mash. Various grains (which may be malted) are used for different varieties, including barley, corn, rye, and wheat. Whisky is typically aged in wooden cask ...
, brandy
Brandy is a liquor produced by distilling wine. Brandy generally contains 35–60% alcohol by volume (70–120 US proof) and is typically consumed as an after-dinner digestif. Some brandies are aged in wooden casks. Others are coloured with ...
, and Campari
Campari () is an Italian alcoholic liqueur, considered an apéritif (20.5%, 21%, 24%, 25%, or 28.5% ABV, depending on the country where it is sold), obtained from the infusion of herbs and fruit (including chinotto and cascarilla) in alcohol a ...
. Soda water may be used to dilute drinks based on cordials such as orange squash
Squash may refer to:
Sports
* Squash (sport), the high-speed racquet sport also known as squash racquets
* Squash (professional wrestling), an extremely one-sided match in professional wrestling
* Squash tennis, a game similar to squash but pla ...
. Soda water is a necessary ingredient in many cocktails, such as whiskey and soda
Scotch and soda is a mixed drink consisting of Scotch whisky and soda water or other sparkling water.
There is no fixed ratio of the ingredients: the amount of water can vary according to taste from a splash to several times that of the whisky. ...
or Campari and soda.
Cooking
Carbonated water is increasingly popular in Western cooking as a substitution for plain water in deep-frying batters to provide a lighter texture to doughs similar to ''tempura
is a typical Japanese dish usually consisting of seafood, meat and vegetables that have been battered and deep fried. The dish was introduced by the Portuguese in Nagasaki through fritter-cooking techniques in the 16th century. The word ''tem ...
''. Kevin Ryan, a food scientist at the University of Illinois at Urbana–Champaign
The University of Illinois Urbana-Champaign (U of I, Illinois, University of Illinois, or UIUC) is a public land-grant research university in Illinois in the twin cities of Champaign and Urbana. It is the flagship institution of the Universit ...
, says the effervescent bubbles when mixed with dough provide a light tempura-like texture, which gives the illusion of being lower calorie than regular frying batters. The lightness is caused by pockets of carbon dioxide gas being introduced into the batter (a process which natural rising using yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitut ...
also creates) and further expanding when cooked.
Stain remover
Since the dissolved gas in carbonated water acts as a temporarysurfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming ...
, it has been recommended as a household remedy for removing stains, particularly those of red wine.
See also
*Premix and postmix
Premix and postmix are two methods of serving – usually carbonated – soft drinks that are alternatives to bottles and cans.
Premix
Premix refers to a ready-mixed, ready-to-drink soft drink that has usually been packaged in 5-gallon stain ...
* Soda jerk
Soda jerk (or soda jerker) is an American term used to refer to a person — typically a young man — who would operate the soda fountain in a drugstore, preparing and serving soda drinks and ice cream sodas. The drinks were made by mixing fl ...
* Sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
* Tonic water
Tonic water (or Indian tonic water) is a carbonated soft drink in which quinine is dissolved. Originally used as a prophylactic against malaria, tonic water usually has a significantly lower quinine content and is consumed for its distinctive b ...
* Limnic eruption
A limnic eruption, also known as a lake overturn, is a very rare type of natural disaster in which dissolved carbon dioxide () suddenly erupts from deep lake waters, forming a gas cloud capable of suffocating wildlife, livestock, and humans. A lim ...
– in deep water lakes, a massive, sudden eruption of dissolved carbon dioxide
References
External links
The Priestley Society
Priestley's paper ''Impregnating Water with Fixed Air'' 1772
{{DEFAULTSORT:Carbonated Water Carbonated drinks English inventions British culture Industrial gases Soft drinks 18th-century inventions Drinks