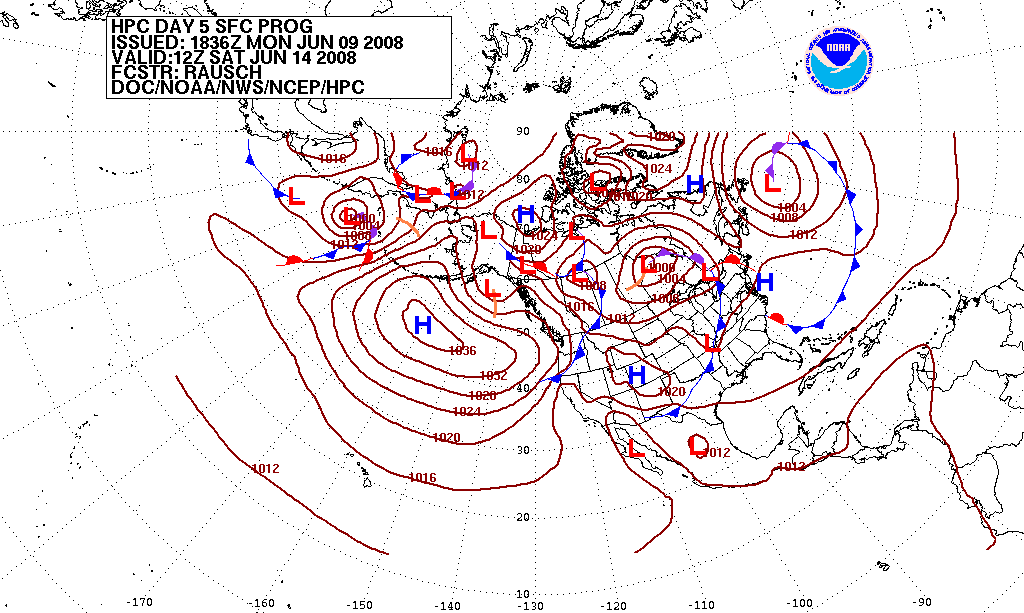
|
Standard Atmospheric Pressure
The standard atmosphere (symbol: atm) is a unit of pressure defined as Pa. It is sometimes used as a ''reference pressure'' or ''standard pressure''. It is approximately equal to Earth's average atmospheric pressure at sea level. History The standard atmosphere was originally defined as the pressure exerted by 760 mm of mercury at and standard gravity (''g''n = ). It was used as a reference condition for physical and chemical properties, and was implicit in the definition of the Celsius temperature scale, which defined as the boiling point of water at this pressure. In 1954, the 10th General Conference on Weights and Measures (CGPM) adopted ''standard atmosphere'' for general use and affirmed its definition of being precisely equal to dynes per square centimetre (). This defined both temperature and pressure independent of the properties of particular substance. In addition, the CGPM noted that there had been some misapprehension that it "led some physicists to believe t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the SI unit of pressure, the pascal (Pa), for example, is one newton per square metre (N/m2); similarly, the pound-force per square inch ( psi) is the traditional unit of pressure in the imperial and U.S. customary systems. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure, and the torr is defined as of this. Manome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bar (unit)
The bar is a metric unit of pressure, but not part of the International System of Units (SI). It is defined as exactly equal to 100,000 Pa (100 kPa), or slightly less than the current average atmospheric pressure on Earth at sea level (approximately 1.013 bar). By the barometric formula, 1 bar is roughly the atmospheric pressure on Earth at an altitude of 111 metres at 15 °C. The bar and the millibar were introduced by the Norwegian meteorologist Vilhelm Bjerknes, who was a founder of the modern practice of weather forecasting. The International System of Units, despite previously mentioning the bar, now omits any mention of it.. The bar has been legally recognised in countries of the European Union since 2004. British Standard BS 350:2004 ''Conversion Factors for Units''. The US National Institute of Standards and Technology (NIST) deprecates its use except for "limited use in meteorology" and lists it as one of several units that "must not be i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmospheric Pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibars, 760 mm Hg, 29.9212 inchesHg, or 14.696 psi.International Civil Aviation Organization. ''Manual of the ICAO Standard Atmosphere'', Doc 7488-CD, Third Edition, 1993. . The atm unit is roughly equivalent to the mean sea-level atmospheric pressure on Earth; that is, the Earth's atmospheric pressure at sea level is approximately 1 atm. In most circumstances, atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. As elevation increases, there is less overlying atmospheric mass, so atmospheric pressure decreases with increasing elevation. Because the atmosphere is thin relative to the Earth's radius—especially the dense atmospheric layer at low altitudes—the E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Temperature And Pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry (IUPAC) and the National Institute of Standards and Technology (NIST), although these are not universally accepted standards. Other organizations have established a variety of alternative definitions for their standard reference conditions. In chemistry, IUPAC changed its definition of standard temperature and pressure in 1982: * Until 1982, STP was defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 1 atm (101.325 kPa). * Since 1982, STP has been defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 105 Pa (100 kPa, 1 bar). STP should not be confused with the standard stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pounds Per Square Foot
The pound per square inch or, more accurately, pound-force per square inch (symbol: lbf/in2; abbreviation: psi) is a unit of pressure or of stress based on avoirdupois units. It is the pressure resulting from a force of one pound-force applied to an area of one square inch. In SI units, 1 psi is approximately equal to 6895 Pa. Pounds per square inch absolute (psia) is used to make it clear that the pressure is relative to a vacuum rather than the ambient atmospheric pressure. Since atmospheric pressure at sea level is around , this will be added to any pressure reading made in air at sea level. The converse is pounds per square inch gauge (psig), indicating that the pressure is relative to atmospheric pressure. For example, a bicycle tire pumped up to 65 psig in a local atmospheric pressure at sea level (14.7 psi) will have a pressure of 79.7 psia (14.7 psi + 65 psi). When gauge pressure is referenced to something other than ambient atmospheric pressure, then the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pounds Per Square Inch
The pound per square inch or, more accurately, pound-force per square inch (symbol: lbf/in2; abbreviation: psi) is a unit of pressure or of stress based on avoirdupois units. It is the pressure resulting from a force of one pound-force applied to an area of one square inch. In SI units, 1 psi is approximately equal to 6895 Pa. Pounds per square inch absolute (psia) is used to make it clear that the pressure is relative to a vacuum rather than the ambient atmospheric pressure. Since atmospheric pressure at sea level is around , this will be added to any pressure reading made in air at sea level. The converse is pounds per square inch gauge (psig), indicating that the pressure is relative to atmospheric pressure. For example, a bicycle tire pumped up to 65 psig in a local atmospheric pressure at sea level (14.7 psi) will have a pressure of 79.7 psia (14.7 psi + 65 psi). When gauge pressure is referenced to something other than ambient atmospheric pressure, then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inch Of Water
Inches of water is a non- SI unit for pressure. It is also given as inches of water gauge (iwg or in.w.g.), inches water column (inch wc, in. WC, " wc, etc. or just wc or WC), inAq, Aq, or inHO. The units are conventionally used for measurement of certain pressure differentials such as small pressure differences across an orifice, or in a pipeline or shaft, or before and after a compressor in an HVAC unit. It is defined as the pressure exerted by a column of water of 1 inch in height at defined conditions. At a temperature of 4 °C (39.2 °F) pure water has its highest density (1000 kg/m3). At that temperature and assuming the standard acceleration of gravity, 1 inAq is approximately 249.082 pascals. Alternative standard conditions in uncommon usage are 60 °F (15,6 °C), or 68 °F (20 °C), and depends on industry standards rather than on international standards. Feet of water is an alternative way to specify pressure as height of a wat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NIST
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical science laboratory programs that include nanoscale science and technology, engineering, information technology, neutron research, material measurement, and physical measurement. From 1901 to 1988, the agency was named the National Bureau of Standards. History Background The Articles of Confederation, ratified by the colonies in 1781, provided: The United States in Congress assembled shall also have the sole and exclusive right and power of regulating the alloy and value of coin struck by their own authority, or by that of the respective states—fixing the standards of weights and measures throughout the United States. Article 1, section 8, of the Constitution of the United States, ratified in 1789, granted these powers to the new Congr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inch Of Mercury
Inch of mercury (inHg and ″Hg) is a non- SI unit of measurement for pressure. It is used for barometric pressure in weather reports, refrigeration and aviation in the United States. It is the pressure exerted by a column of mercury in height at the standard acceleration of gravity. Conversion to metric units depends on the temperature of mercury, and hence its density; typical conversion factors are: In older literature, an "inch of mercury" is based on the height of a column of mercury at .Barry N. Taylor, ''Guide for the Use of the International System of Units (SI),'' 1995, NIST Special Publication 811, Appendix /ref> :1 inHg60 °F = In Imperial units: 1 inHg60 °F = 0.489 771 Pounds per square inch, psi, or 2.041 771 inHg60 °F = 1 psi. Applications Aircraft and automobiles Aircraft altimeters measure the relative pressure difference between the lower ambient pressure at altitude and a calibrated reading on the ground. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Torr
The torr (symbol: Torr) is a unit of pressure based on an absolute scale, defined as exactly of a standard atmosphere (). Thus one torr is exactly (≈ ). Historically, one torr was intended to be the same as one "millimeter of mercury", but subsequent redefinitions of the two units made them slightly different (by less than ). The torr is not part of the International System of Units (SI). It is often combined with the metric prefix milli to name one millitorr (mTorr) or 0.001 Torr. The unit was named after Evangelista Torricelli, an Italian physicist and mathematician who discovered the principle of the barometer in 1644. Nomenclature and common errors The unit name ''torr'' is written in lower case, while its symbol ("Torr") is always written with upper-case initial; including in combinations with prefixes and other unit symbols, as in "mTorr" (millitorr) or "Torr⋅L/s" (torr-litres per second). The symbol (uppercase) should be used with prefix symbols ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Millimetre Of Mercury
A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly pascals. It is denoted mmHg or mm Hg. Although not an SI unit, the millimetre of mercury is still routinely used in medicine, meteorology, aviation, and many other scientific fields. One millimetre of mercury is approximately 1 Torr, which is of standard atmospheric pressure ( ≈ ). Although the two units are not equal, the relative difference (less than ) is negligible for most practical uses. History For much of human history, the pressure of gases like air was ignored, denied, or taken for granted, but as early as the 6th century BC, Greek philosopher Anaximenes of Miletus claimed that all things are made of air that is simply changed by varying levels of pressure. He could observe water evaporating, changing to a gas, and felt that this applied even to solid matter. Mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vienna Standard Mean Ocean Water
Vienna Standard Mean Ocean Water (VSMOW) is an isotopic standard for water. Despite the name, VSMOW is pure water with no salt or other chemicals found in the oceans. The VSMOW standard was promulgated by the International Atomic Energy Agency (based in Vienna) in 1968, and since 1993 continues to be evaluated and studied by the IAEA along with the European Institute for Reference Materials and Measurements and the American National Institute of Standards and Technology. The standard includes both the established values of stable isotopes found in waters and calibration materials provided for standardization and interlaboratory comparisons of instruments used to measure these values in experimental materials. The designation ''ocean water'' refers to water molecules collected directly from the ocean, rather than from other points in the water cycle (e.g., rain, snow, river or lake water). Water from different points in the water cycle contains molecules with differing ratios of i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |