Sarin on:
[Wikipedia]
[Google]
[Amazon]
Sarin (
Emergency Response Safety and Health Database. National Institute for Occupational Safety and Health. Accessed April 20, 2009. A colourless, odourless
 Like some other nerve agents that affect the
Like some other nerve agents that affect the
 The second process, known as the "Di-Di" process, uses equal quantities of
The second process, known as the "Di-Di" process, uses equal quantities of 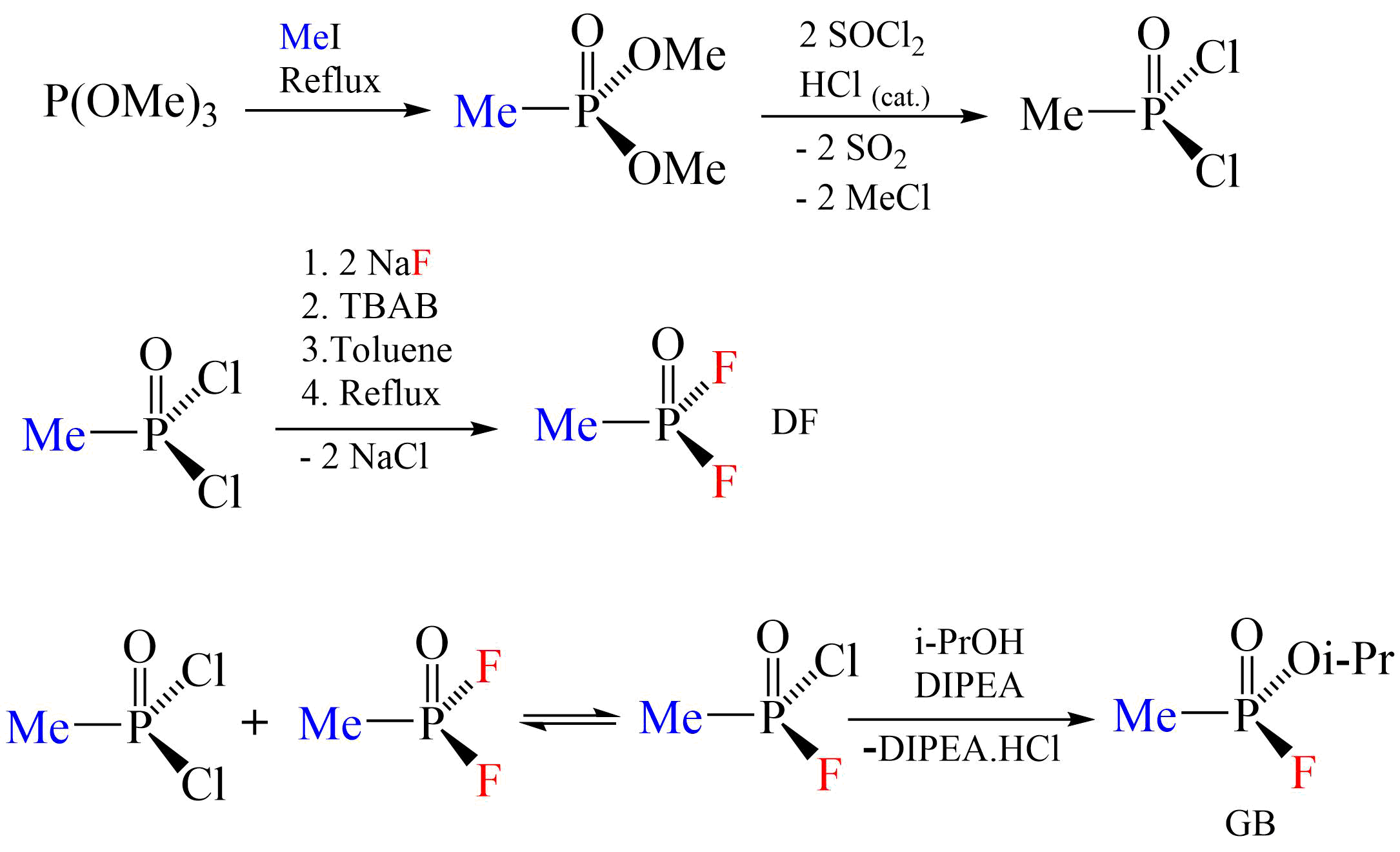 As both reactions leave considerable acid in the product, sarin produced in bulk by these methods has a short half life without further processing, and would be corrosive to containers and damaging to weapons systems. Various methods have been tried to resolve these problems. In addition to industrial
As both reactions leave considerable acid in the product, sarin produced in bulk by these methods has a short half life without further processing, and would be corrosive to containers and damaging to weapons systems. Various methods have been tried to resolve these problems. In addition to industrial (CH3)2CHO- + CH3P(O)FOCH(CH3)2 -> CH3P(O)(OCH(CH3)2)2 + F-
This chemical degrades into isopropyl methylphosphonic acid.
 The most important chemical reactions of phosphoryl halides is the hydrolysis of the bond between phosphorus and the fluoride. This P-F bond is easily broken by nucleophilic agents, such as water and
The most important chemical reactions of phosphoryl halides is the hydrolysis of the bond between phosphorus and the fluoride. This P-F bond is easily broken by nucleophilic agents, such as water and
 * 1950s (early):
* 1950s (early):
Material Safety Data Sheet
CDC Sarin fact sheet
{{Phosphorus compounds Acetylcholinesterase inhibitors Chemical weapons of the United States Cold War weapons of the Soviet Union G-series nerve agents German chemical weapons program German inventions of the Nazi period Isopropyl esters Methylphosphonofluoridates Soviet chemical weapons program Substances discovered in the 1930s Toxicology United Kingdom chemical weapons program
NATO
The North Atlantic Treaty Organization (NATO, ; french: Organisation du traité de l'Atlantique nord, ), also called the North Atlantic Alliance, is an intergovernmental military alliance between 30 member states – 28 European and two No ...
designation GB G-series,_"B".html" ;"title="Nerve_agent#G-series.html" ;"title="hort for Nerve agent#G-series">G-series, "B"">Nerve_agent#G-series.html" ;"title="hort for Nerve agent#G-series">G-series, "B" is an extremely toxic synthetic organophosphorus compound.Sarin (GB)Emergency Response Safety and Health Database. National Institute for Occupational Safety and Health. Accessed April 20, 2009. A colourless, odourless
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
, it is used as a chemical weapon
A chemical weapon (CW) is a specialized munition that uses chemicals formulated to inflict death or harm on humans. According to the Organisation for the Prohibition of Chemical Weapons (OPCW), this can be any chemical compound intended as a ...
due to its extreme potency as a nerve agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that ...
. Exposure is lethal even at very low concentrations, where death can occur within one to ten minutes after direct inhalation of a lethal dose, due to suffocation from respiratory paralysis, unless antidotes are quickly administered. People who absorb a non-lethal dose and do not receive immediate medical treatment may suffer permanent neurological damage.
Sarin is widely considered a weapon of mass destruction
A weapon of mass destruction (WMD) is a chemical, biological, radiological, nuclear, or any other weapon that can kill and bring significant harm to numerous individuals or cause great damage to artificial structures (e.g., buildings), natura ...
. Production and stockpiling of sarin was outlawed as of April 1997 by the Chemical Weapons Convention
The Chemical Weapons Convention (CWC), officially the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction, is an arms control treaty administered by the Organisation for ...
of 1993, and it is classified as a Schedule 1 substance.
Health effects
neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Part ...
, sarin attacks the nervous system
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes th ...
by interfering with the degradation of the neurotransmitter acetylcholine at neuromuscular junction
A neuromuscular junction (or myoneural junction) is a chemical synapse between a motor neuron and a muscle fiber.
It allows the motor neuron to transmit a signal to the muscle fiber, causing muscle contraction.
Muscles require innervation to ...
s. Death will usually occur as a result of asphyxia
Asphyxia or asphyxiation is a condition of deficient supply of oxygen to the body which arises from abnormal breathing. Asphyxia causes generalized hypoxia, which affects primarily the tissues and organs. There are many circumstances that can i ...
due to the inability to control the muscles involved in breathing.
Initial symptoms following exposure to sarin are a runny nose
Rhinorrhea, rhinorrhoea, or informally runny nose is the free discharge of a thin mucus fluid from the nose; it is a common condition. It is a common symptom of allergies (hay fever) or certain viral infections, such as the common cold or COVID-1 ...
, tightness in the chest, and constriction of the pupils. Soon after, the person will have difficulty breathing and they will experience nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. While not painful, it can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the ...
and drooling. As they continue to lose control of bodily functions, they may vomit, defecate, and urinate. This phase is followed by twitching and jerking. Ultimately, the person becomes comatose and suffocates in a series of convulsive spasm
A spasm is a sudden involuntary contraction of a muscle, a group of muscles, or a hollow organ such as the bladder.
A spasmodic muscle contraction may be caused by many medical conditions, including dystonia. Most commonly, it is a muscle c ...
s. Moreover, common mnemonics for the symptomatology of organophosphate poisoning, including sarin, are the "killer Bs" of bronchorrhea Bronchorrhea is the production of more than 100 mL per day of watery sputum. Chronic bronchitis is a common cause, but it may also be caused by asthma, pulmonary contusion,
bronchiectasis, tuberculosis, cancer, scorpion stings, severe hypothermia ...
and bronchospasm
Bronchospasm or a bronchial spasm is a sudden constriction of the muscles in the walls of the bronchioles. It is caused by the release (degranulation) of substances from mast cells or basophils under the influence of anaphylatoxins. It causes di ...
because they are the leading cause of death, and SLUDGE
Sludge is a semi-solid slurry that can be produced from a range of industrial processes, from water treatment, wastewater treatment or on-site sanitation systems. For example, it can be produced as a settled suspension obtained from conventiona ...
– salivation, lacrimation
Tears are a clear liquid secreted by the lacrimal glands (tear gland) found in the eyes of all land mammals. Tears are made up of water, electrolytes, proteins, lipids, and mucins that form layers on the surface of eyes. The different types of ...
, urination, defecation, gastrointestinal distress, and emesis (vomiting). Death may follow in one to ten minutes after direct inhalation.
Sarin has a high volatility (ease with which a liquid can turn into vapour) relative to similar nerve agents, making inhalation very easy, and may even absorb through the skin. A person's clothing can release sarin for about 30 minutes after it has come in contact with sarin gas, which can lead to exposure of other people.
Management
Treatment measures have been described. Treatment is typically with theantidote
An antidote is a substance that can counteract a form of poisoning. The term ultimately derives from the Greek term φάρμακον ἀντίδοτον ''(pharmakon) antidoton'', "(medicine) given as a remedy". Antidotes for anticoagulants are s ...
s atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given i ...
and pralidoxime
Pralidoxime (2-pyridine aldoxime methyl chloride) or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to treat organophosphat ...
. Atropine, an antagonist
An antagonist is a character in a story who is presented as the chief foe of the protagonist.
Etymology
The English word antagonist comes from the Greek ἀνταγωνιστής – ''antagonistēs'', "opponent, competitor, villain, enemy, riv ...
to muscarinic acetylcholine receptor
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-rece ...
s, is given to treat the physiological symptoms of poisoning. Since muscular response to acetylcholine is mediated through nicotinic acetylcholine receptor
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral ne ...
s, atropine does not counteract the muscular symptoms. Pralidoxime can regenerate cholinesterase
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
: an acylcholine + H2O = choline + a carboxylate
Several of these serve as neurotransmitters ...
s if administered within approximately five hours. Biperiden
Biperiden, sold under the brand name Akineton among others, is a medication used to treat Parkinson disease and certain drug-induced movement disorders. It is not recommended for tardive dyskinesias. It is taken by mouth, injection into a vein, ...
, a synthetic acetylcholine antagonist, has been suggested as an alternative to atropine due to its better blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of ...
penetration and higher efficacy.
Mechanism of action
Sarin is a potent inhibitor of acetylcholinesterase, an enzyme that degrades theneurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Part ...
after it is released into the synaptic cleft
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous syste ...
. In vertebrates, acetylcholine is the neurotransmitter used at the neuromuscular junction, where signals are transmitted between neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. N ...
s from the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
to muscle fibres. Normally, acetylcholine is released from the neuron to stimulate the muscle, after which it is degraded by acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme
Enzymes () are proteins that a ...
, allowing the muscle to relax. A build-up of acetylcholine in the synaptic cleft
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous syste ...
, due to the inhibition of acetylcholinesterase, means the neurotransmitter continues to act on the muscle fibre, so that any nerve impulses are effectively continually transmitted.
Sarin acts on acetylcholinesterase by forming a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
with the particular serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
residue at the active site. Fluoride is the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
, and the resulting organo-phosphoester is robust and biologically inactive.
Its mechanism of action resembles that of some commonly used insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to b ...
s, such as malathion
Malathion is an organophosphate insecticide which acts as an acetylcholinesterase inhibitor. In the USSR, it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion.
Pesticide use
Malathion is a pesti ...
. In terms of biological activity, it resembles carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
insecticides, such as Sevin, and the medicines pyridostigmine
Pyridostigmine is a medication used to treat myasthenia gravis and underactive bladder. It is also used together with atropine to end the effects of neuromuscular blocking medication of the non-depolarizing type. It is typically given by mouth ...
, neostigmine
Neostigmine, sold under the brand name Bloxiverz, among others, is a medication used to treat myasthenia gravis, Ogilvie syndrome, and urinary retention without the presence of a blockage. It is also used in anaesthesia to end the effects of n ...
, and physostigmine
Physostigmine (also known as eserine from ''éséré'', the West African name for the Calabar bean) is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and ...
.
Diagnostic tests
Controlled studies in healthy men have shown that a nontoxic 0.43 mg oral dose administered in several portions over a 3-day interval caused average maximum depressions of 22 and 30%, respectively, in plasma and erythrocyte acetylcholinesterase levels. A single acute 0.5 mg dose caused mild symptoms of intoxication and an average reduction of 38% in both measures of acetylcholinesterase activity. Sarin in blood is rapidly degraded either ''in vivo'' or ''in vitro''. Its primary inactivemetabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
s have ''in vivo'' serum half-lives of approximately 24 hours. The serum level of unbound isopropyl methylphosphonic acid (IMPA), a sarin hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
product, ranged from 2–135 μg/L in survivors of a terrorist attack during the first four hours post-exposure. Sarin or its metabolites may be determined in blood or urine by gas or liquid chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
, while acetylcholinesterase activity is usually measured by enzymatic methods.
A newer method called "fluoride regeneration" or "fluoride reactivation" detects the presence of nerve agents for a longer period after exposure than the methods described above. Fluoride reactivation is a technique that has been explored since at least the early 2000s. This technique obviates some of the deficiencies of older procedures. Sarin not only reacts with the water in the blood plasma through hydrolysis (forming so-called 'free metabolites'), but also reacts with various proteins to form 'protein adducts'. These protein adducts are not so easily removed from the body, and remain for a longer period of time than the free metabolites. One clear advantage of this process is that the period, post-exposure, for determination of sarin exposure is much longer, possibly five to eight weeks according to at least one study.
Toxicity
As a nerve gas, sarin in its purest form is estimated to be 26 times more deadly thancyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
. The LD50 of subcutaneously injected sarin in mice is 172 μg/kg.
Sarin is highly toxic, whether by contact with the skin or breathed in. The toxicity of sarin in humans is largely based on calculations from studies with animals. The lethal concentration of sarin in air is approximately 28–35 mg per cubic meter per minute for a two-minute exposure time by a healthy adult breathing normally (exchanging 15 liters of air per minute, lower 28 mg/m3 value is for general population). This number represents the estimated lethal concentration for 50% of exposed victims, the LCt50 value. The LCt95 or LCt100 value is estimated to be 40–83 mg per cubic meter for exposure time of two minutes. Calculating effects for different exposure times and concentrations requires following specific toxic load models. In general, brief exposures to higher concentrations are more lethal than comparable long time exposures to low concentrations. There are many ways to make relative comparisons between toxic substances. The list below compares sarin to some current and historic chemical warfare agents, with a direct comparison to the respiratory LCt50:
* Hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
, 2,860 mg·min/m3 – Sarin is 81 times more lethal
* Phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ...
, 1,500 mg·min/m3 – Sarin is 43 times more lethal
* Sulfur mustard
Mustard gas or sulfur mustard is a chemical compound belonging to a family of cytotoxic and blister agents known as mustard agents. The name ''mustard gas'' is technically incorrect: the substance, when dispersed, is often not actually a gas, b ...
, 1,000 mg·min/m3 – Sarin is 28 times more lethal
* Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
, 19,000 mg·min/m3 – Sarin is 543 times more lethal
Production and structure
Sarin is achiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
molecule because it has four chemically distinct substituents
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
attached to the tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
phosphorus center. The ''SP ''form (the (–) optical isomer) is the more active enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
due to its greater binding affinity
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a mol ...
to acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme
Enzymes () are proteins that a ...
. The P-F bond is easily broken by nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
agents, such as water and hydroxide. At high p''H'', sarin decomposes rapidly to nontoxic phosphonic acid derivatives.
It is almost always manufactured as a racemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
(a 1:1 mixture of its enantiomeric forms) as this involves a much simpler synthetic process whilst providing an adequate weapon.
A number of production pathways can be used to create sarin. The final reaction typically involves attachment of the isopropoxy group to the phosphorus with an alcoholysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
with isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
. Two variants of this process are common. One is the reaction of methylphosphonyl difluoride
Methylphosphonyl difluoride (DF), also known as EA-1251 or difluoro, is a chemical weapon precursor. Its chemical formula is CH3POF2. It is a List of Schedule 1 substances (CWC), Schedule 1 substance under the Chemical Weapons Convention. It is ...
with isopropyl alcohol, which produces a racemic mixture of sarin enantiomers with hydrofluoric acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include th ...
as a byproduct:
: The second process, known as the "Di-Di" process, uses equal quantities of
The second process, known as the "Di-Di" process, uses equal quantities of methylphosphonyl difluoride
Methylphosphonyl difluoride (DF), also known as EA-1251 or difluoro, is a chemical weapon precursor. Its chemical formula is CH3POF2. It is a List of Schedule 1 substances (CWC), Schedule 1 substance under the Chemical Weapons Convention. It is ...
(Difluoro) and methylphosphonyl dichloride
Methylphosphonyl dichloride (DC) or dichloro is an organophosphorus compound. It has a number of commercial uses but is most notable as being a precursor to several chemical weapons agents. It is a white crystalline solid that melts slightly abov ...
(Dichloro), rather than just the difluoride. This reaction also gives sarin, but hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
as a byproduct instead. The Di-Di process was used by the United States for the production of its unitary sarin stockpile.
The scheme below shows a generic example of the Di-Di process; in reality, the selection of reagents and reaction conditions dictate both product structure and yield. The choice of enantiomer of the mixed chloro fluoro intermediate displayed in the diagram is arbitrary, but the final substitution is selective for chloro over fluoro as the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
. Inert atmosphere and anhydrous conditions ( Schlenk techniques) are used for synthesis of sarin and other organophosphates.
 As both reactions leave considerable acid in the product, sarin produced in bulk by these methods has a short half life without further processing, and would be corrosive to containers and damaging to weapons systems. Various methods have been tried to resolve these problems. In addition to industrial
As both reactions leave considerable acid in the product, sarin produced in bulk by these methods has a short half life without further processing, and would be corrosive to containers and damaging to weapons systems. Various methods have been tried to resolve these problems. In addition to industrial refining
{{Unreferenced, date=December 2009
Refining (also perhaps called by the mathematical term affining) is the process of purification of a (1) substance or a (2) form. The term is usually used of a natural resource that is almost in a usable form, b ...
techniques to purify the chemical itself, various additives have been tried to combat the effects of the acid, such as:
* Tributylamine
Tributylamine (TBA) is an organic compound with the molecular formula (C4H9)3N. It is a colorless liquid with an amine-like odor.
Uses
Tributylamine is used as a catalyst (proton acceptor) and as a solvent in organic syntheses and polymerization ...
was added to US sarin produced at Rocky Mountain Arsenal
The Rocky Mountain Arsenal was a United States chemical weapons manufacturing center located in the Denver Metropolitan Area in Commerce City, Colorado. The site was completed December 1942, operated by the United States Army throughout the late ...
.
* Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
was added to UK sarin, with relatively poor success. The Aum Shinrikyo
, formerly , is a Japanese doomsday cult founded by Shoko Asahara in 1987. It carried out the deadly Tokyo subway sarin attack in 1995 and was found to have been responsible for the Matsumoto sarin attack the previous year.
The group says tha ...
cult experimented with triethylamine as well.
* ''N'',''N''-Diethylaniline was used by Aum Shinrikyo for acid reduction.
* ''N'',''N′''-Diisopropylcarbodimide was added to sarin produced at Rocky Mountain Arsenal to combat corrosion.
* Isopropylamine
Isopropylamine (monoisopropyl amine, MIPA, 2-Propylamine) is an organic compound, an amine. It is a hygroscopic colorless liquid with ammonia-like odor. It is miscible with water and flammable. It is a valuable intermediate in chemical industry. ...
was included as part of the M687
The M687 was an American 155 mm binary sarin chemical artillery shell. The design was standardized in 1976 and production began on December 16, 1987 at Pine Bluff Arsenal, Pine Bluff, Arkansas. Production was halted three years later, following th ...
155 mm field artillery shell, which was a binary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two digits (0 and 1)
* Binary function, a function that takes two arguments
* Binary operation, a mathematical operation that t ...
sarin weapon system developed by the US Army.
Another byproduct of these two chemical processes is diisopropyl methylphosphonate
Diisopropyl methylphosphonate (DIMP), also known as diisopropyl methane-phosphonate and phosphonic acid and methyl-bis-(1-methylethyl)ester, is a chemical by-product in the production of sarin gas.
DIMP is a colorless liquid that has been shown ...
, formed when a second isopropyl alcohol reacts with the sarin itself and from disproportionation of sarin, when distilled incorrectly. The factor of its formation in esterification is that as the concentration of DF-DCl decreases, the concentration of sarin increases, the probability of DIMP formation is greater. DIMP is a natural impurity of sarin, that is almost impossible to be eliminated, mathematically, when the reaction is a 1 mol-1 mol "one-stream".
Degradation and shelf life
 The most important chemical reactions of phosphoryl halides is the hydrolysis of the bond between phosphorus and the fluoride. This P-F bond is easily broken by nucleophilic agents, such as water and
The most important chemical reactions of phosphoryl halides is the hydrolysis of the bond between phosphorus and the fluoride. This P-F bond is easily broken by nucleophilic agents, such as water and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
. At high pH, sarin decomposes rapidly to nontoxic phosphonic acid derivatives. The initial breakdown of sarin is into isopropyl methylphosphonic acid (IMPA), a chemical that is not commonly found in nature except as a breakdown product of sarin (this is useful for detecting the recent deployment of sarin as a weapon). IMPA then degrades into methylphosphonic acid
Methylphosphonic acid is an organophosphorus compound with the chemical formula CH3P(O)(OH)2. The phosphorus center is tetrahedral and is bonded to a methyl group, two OH groups and an oxygen. Methylphosphonic acid is a white, non-volatile solid t ...
(MPA), which can also be produced by other organophosphates.
Sarin with residual acid degrades after a period of several weeks to several months. The shelf life can be shortened by impurities in precursor materials. According to the CIA
The Central Intelligence Agency (CIA ), known informally as the Agency and historically as the Company, is a civilian intelligence agency, foreign intelligence service of the federal government of the United States, officially tasked with gat ...
, some Iraqi sarin had a shelf life of only a few weeks, owing mostly to impure precursors.
Along with nerve agents such as tabun and VX, sarin can have a short shelf life. Therefore, it is usually stored as two separate precursors that produce sarin when combined. Sarin's shelf life can be extended by increasing the purity of the precursor and intermediates and incorporating stabilizers such as tributylamine
Tributylamine (TBA) is an organic compound with the molecular formula (C4H9)3N. It is a colorless liquid with an amine-like odor.
Uses
Tributylamine is used as a catalyst (proton acceptor) and as a solvent in organic syntheses and polymerization ...
. In some formulations, tributylamine is replaced by diisopropylcarbodiimide
''N'',''N′''-Diisopropylcarbodiimide is a carbodiimide used in peptide synthesis. As a liquid, it is easier to handle than the commonly used ''N'',''N′''-dicyclohexylcarbodiimide, a waxy solid. In addition, ''N'',''N′''-diisopr ...
(DIC), allowing sarin to be stored in aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
casings. In binary chemical weapon
__NOTOC__
Binary chemical weapons or munitions are chemical weapons which contain the toxic agent in its active state as chemical precursors that are significantly less toxic than the agent. This improves the safety of storing, transporting, and di ...
s, the two precursors are stored separately in the same shell
Shell may refer to:
Architecture and design
* Shell (structure), a thin structure
** Concrete shell, a thin shell of concrete, usually with no interior columns or exterior buttresses
** Thin-shell structure
Science Biology
* Seashell, a hard ou ...
and mixed to form the agent immediately before or when the shell is in flight. This approach has the dual benefit of solving the stability issue and increasing the safety of sarin munitions.
History
Sarin was discovered in 1938 inWuppertal
Wuppertal (; "''Wupper Dale''") is, with a population of approximately 355,000, the seventh-largest city in North Rhine-Westphalia as well as the 17th-largest city of Germany. It was founded in 1929 by the merger of the cities and to ...
-Elberfeld in Germany by scientists at IG Farben
Interessengemeinschaft Farbenindustrie AG (), commonly known as IG Farben (German for 'IG Dyestuffs'), was a German chemical and pharmaceutical conglomerate (company), conglomerate. Formed in 1925 from a merger of six chemical companies—BASF, ...
who were attempting to create stronger pesticides; it is the most toxic of the four G-Series nerve agents G series may refer to:
Transportation
*G series (Toronto subway), a line of subway cars
* Chevrolet G-series vans
*G-series trains, the designation for the fastest long-distance trains in China
*Infiniti G-series (Q40/Q60), a line of luxury sports ...
made by Germany. The compound, which followed the discovery of the nerve agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that ...
tabun, was named in honor of its discoverers: chemist Gerhard Schrader, chemist Otto Ambros, chemist , and from Heereswaffenamt
''Waffenamt'' (WaA) was the German Army Weapons Agency. It was the centre for research and development of the Weimar Republic and later the Third Reich for weapons, ammunition and army equipment to the German Reichswehr and then Wehrmacht
...
Hans-Jürgen von der Linde.
Use as a weapon
In mid-1939, the formula for the agent was passed to thechemical warfare
Chemical warfare (CW) involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare, biological warfare and radiological warfare, which together make up CBRN, the military acronym ...
section of the German Army Weapons Office, which ordered that it be brought into mass production for wartime use. Pilot plants were built, and a high-production facility was under construction (but was not finished) by the end of World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposin ...
. Estimates for total sarin production by Nazi Germany
Nazi Germany (lit. "National Socialist State"), ' (lit. "Nazi State") for short; also ' (lit. "National Socialist Germany") (officially known as the German Reich from 1933 until 1943, and the Greater German Reich from 1943 to 1945) was ...
range from 500 kg to 10 tons.
Though sarin, tabun and soman
Soman (or GD, EA 1210, Zoman, PFMP, A-255, systematic name: ''O''-pinacolyl methylphosphonofluoridate) is an extremely toxic chemical substance. It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiti ...
were incorporated into artillery
Artillery is a class of heavy military ranged weapons that launch munitions far beyond the range and power of infantry firearms. Early artillery development focused on the ability to breach defensive walls and fortifications during siege ...
shells, Germany did not use nerve agents against Allied
An alliance is a relationship among people, groups, or states that have joined together for mutual benefit or to achieve some common purpose, whether or not explicit agreement has been worked out among them. Members of an alliance are called ...
targets. Adolf Hitler
Adolf Hitler (; 20 April 188930 April 1945) was an Austrian-born German politician who was dictator of Nazi Germany, Germany from 1933 until Death of Adolf Hitler, his death in 1945. Adolf Hitler's rise to power, He rose to power as the le ...
refused to initiate the use of gases such as sarin as weapons.
 * 1950s (early):
* 1950s (early): NATO
The North Atlantic Treaty Organization (NATO, ; french: Organisation du traité de l'Atlantique nord, ), also called the North Atlantic Alliance, is an intergovernmental military alliance between 30 member states – 28 European and two No ...
adopted sarin as a standard chemical weapon, and both the USSR and the United States produced sarin for military purposes.
* 1953: 20-year-old Ronald Maddison
Leading Aircraftman Ronald George Maddison (23 January 1933 – 6 May 1953) was a twenty-year-old Royal Air Force engineer who was unlawfully killed as the result of exposure to nerve agents while acting as a voluntary test subject at Porton Down ...
, a Royal Air Force
The Royal Air Force (RAF) is the United Kingdom's air and space force. It was formed towards the end of the First World War on 1 April 1918, becoming the first independent air force in the world, by regrouping the Royal Flying Corps (RFC) and ...
engineer from Consett
Consett is a town in County Durham, England, about south-west of Newcastle upon Tyne. It had a population of 27,394 in 2001 and an estimate of 25,812 in 2019.
History
Consett sits high on the edge of the Pennines. Its' name originates in the ...
, County Durham
County Durham ( ), officially simply Durham,UK General Acts 1997 c. 23Lieutenancies Act 1997 Schedule 1(3). From legislation.gov.uk, retrieved 6 April 2022. is a ceremonial county in North East England.North East Assembly �About North East E ...
, died in human testing of sarin at the Porton Down
Porton Down is a science park in Wiltshire, England, just northeast of the village of Porton, near Salisbury. It is home to two British government facilities: a site of the Ministry of Defence's Defence Science and Technology Laboratory (Dstl ...
chemical warfare testing facility in Wiltshire
Wiltshire (; abbreviated Wilts) is a historic and ceremonial county in South West England with an area of . It is landlocked and borders the counties of Dorset to the southwest, Somerset to the west, Hampshire to the southeast, Gloucestershire ...
, England. Ten days after his death an inquest
An inquest is a judicial inquiry in common law jurisdictions, particularly one held to determine the cause of a person's death. Conducted by a judge, jury, or government official, an inquest may or may not require an autopsy carried out by a coro ...
was held in secret which returned a verdict of misadventure. In 2004, the inquest was reopened and, after a 64-day inquest hearing, the jury ruled that Maddison had been unlawfully killed by the "application of a nerve agent in a non-therapeutic experiment".
* 1957: Regular production of sarin chemical weapons ceased in the United States, though existing stocks of bulk sarin were re-distilled until 1970.
* 1976: Chile's intelligence service, DINA
Dina ( ar, دينا, he, דִּינָה, also spelled Dinah, Dena, Deena) is a female given name.
Women
* Dina bint Abdul-Hamid (1929–2019), Queen consort of Jordan, first wife of King Hussein
* Princess Dina Mired of Jordan (born 1965), Princ ...
, assigned biochemist Eugenio Berríos
Eugenio Berríos Sagredo (November 14, 1947 – November 15, 1992) was a Chilean biochemist who worked for the Dirección de Inteligencia Nacional (DINA).
Berríos was charged with carrying out '' Proyecto Andrea'' in which Pinochet ordered ...
to develop Sarin gas within its program '' Proyecto Andrea'', to be used as a weapon against its opponents. One of DINA's goals was to package it in spray cans for easy use, which, according to testimony by former DINA agent Michael Townley
Michael Vernon Townley (born December 5, 1942, in Waterloo, Iowa) is an American-born former agent of the Dirección de Inteligencia Nacional (DINA), the secret police of Chile during the regime of Augusto Pinochet. In 1978, Townley pled guilty t ...
, was one of the planned procedures in the 1976 assassination of Orlando Letelier
On 21 September 1976, Orlando Letelier, a leading opponent of Chilean dictator Augusto Pinochet, was assassinated by car bombing, in Washington, D.C. Letelier, who was living in exile in the United States, was killed along with his work colleagu ...
. Berríos later testified that it was used in a number of assassinations and it was planned to be used to kill inhabitants, through poisoning the water supply of Argentine
Argentines (mistakenly translated Argentineans in the past; in Spanish (masculine) or (feminine)) are people identified with the country of Argentina. This connection may be residential, legal, historical or cultural. For most Argentines, s ...
capital Buenos Aires
Buenos Aires ( or ; ), officially the Autonomous City of Buenos Aires ( es, link=no, Ciudad Autónoma de Buenos Aires), is the capital and primate city of Argentina. The city is located on the western shore of the Río de la Plata, on South ...
, in case Operation Soberanía
Operación Soberanía (Operation Sovereignty) was a planned Argentine military invasion of Chile due to the Beagle conflict. The invasion was initiated on 22 December 1978 but was halted after a few hours and Argentine forces retreated from the con ...
took place.
* March 1988: Halabja chemical attack
The Halabja massacre ( ku, Kêmyabarana Helebce کیمیابارانی ھەڵەبجە), also known as the Halabja chemical attack, was a massacre of Kurdish people that took place on 16 March 1988, during the closing days of the Iran–Iraq War ...
; Over two days in March, the ethnic Kurdish
Kurdish may refer to:
*Kurds or Kurdish people
*Kurdish languages
*Kurdish alphabets
*Kurdistan, the land of the Kurdish people which includes:
**Southern Kurdistan
**Eastern Kurdistan
**Northern Kurdistan
**Western Kurdistan
See also
* Kurd (dis ...
city of Halabja
Halabja ( ku, هەڵەبجە, Helebce, ) is a city in the Kurdistan Region of Iraq and the capital of Halabja Governorate, located about northeast of Baghdad and from the Iranian border.
The city lies at the base of what is often referred to ...
in northern Iraq (population 70,000) was bombarded by Saddam Hussein
Saddam Hussein ( ; ar, صدام حسين, Ṣaddām Ḥusayn; 28 April 1937 – 30 December 2006) was an Iraqi politician who served as the fifth president of Iraq from 16 July 1979 until 9 April 2003. A leading member of the revolution ...
's Iraqi Air Force
The Iraqi Air Force (IQAF or IrAF) ( ar, القوات الجوية العراقية, Al Quwwat al Jawwiyah al Iraqiyyah}) is the aerial warfare service branch of the Iraqi Armed Forces. It is responsible for the defense of Iraqi airspace as well ...
jets with chemical bombs including sarin. An estimated 5,000 people died, almost all civilians.
* April 1988: Sarin was used four times against Iranian soldiers at the end of the Iran–Iraq War
The Iran–Iraq War was an armed conflict between Iran and Iraq that lasted from September 1980 to August 1988. It began with the Iraqi invasion of Iran and lasted for almost eight years, until the acceptance of United Nations Security Council ...
, helping Iraqi forces to retake control of the al-Faw Peninsula during the Second Battle of al-Faw
The Second Battle of al-Faw (also known as the Operation Ramadan Mubarak (Blessed Ramadan)), fought on 17 April 1988, was a major battle of the Iran–Iraq War. After their defeat at the First Battle of al-Faw two years earlier, the newly restru ...
.
* 1993: The United Nations Chemical Weapons Convention
The Chemical Weapons Convention (CWC), officially the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction, is an arms control treaty administered by the Organisation for ...
was signed by 162 member countries, banning the production and stockpiling of many chemical weapons, including sarin. It went into effect on April 29, 1997, and called for the complete destruction of all specified stockpiles of chemical weapons by April 2007. When the convention entered force, the parties declared worldwide stockpiles of 15,047 tonnes of sarin. As of November 28th, 2019, 98% of the stockpiles have been destroyed.
*1990: Kosovo student poisoning In March 1990, several months after the unilateral move by the Serbian government to segregate schools throughout Kosovo
Kosovo ( sq, Kosova or ; sr-Cyrl, Косово ), officially the Republic of Kosovo ( sq, Republika e Kosovës, links=no; sr, Република Косово, Republika Kosovo, links=no), is a partially recognised state in Southeast Euro ...
, a mysterious illness – massive poisoning of mostly school children appeared. The first victims to suffer this disease were students, who seasoned the most terrific nightmare of their lives. Schoolchildren could detect a "white powder" on their desks. If they poked it, they quickly developed symptoms: First froth around the mouth and then cramps and fainting. Many schools from each corner of Kosovo began to report such happenings and from the first day on the absence of pupils in schools started to increase.Hyseni, Halim. E verteta per Helmimet ne Kosovë On 1 August 1990, French doctor Bernard Benedetti, in an interview for “ The LaCourse” newspaper, claimed that he secretly entered a hospital in Pristina
Pristina, ; sr, / (, ) is the capital and largest city of Kosovo. The city's municipal boundaries in Pristina District form the largest urban center in Kosovo. After Tirana, Pristina has the second largest population of ethnic Albanians and ...
and obtained blood samples from 150 patients. The analyses were done in two laboratories in Paris. According to Dr. Benedetti, those patients were poisoned. When Mr. Benedetti visited Kosovo
Kosovo ( sq, Kosova or ; sr-Cyrl, Косово ), officially the Republic of Kosovo ( sq, Republika e Kosovës, links=no; sr, Република Косово, Republika Kosovo, links=no), is a partially recognised state in Southeast Euro ...
again in 2000, he confirmed the results of the 1990s tests. According to him, publication of the results was stopped by the French government in an attempt to preserve diplomatic relations with Serbia. Another group called the Commission of Geneva
Commission or commissioning may refer to:
Business and contracting
* Commission (remuneration), a form of payment to an agent for services rendered
** Commission (art), the purchase or the creation of a piece of art most often on behalf of anothe ...
was sent in Kosovo. This group was made up of Charles Graves, Verena Graf
Verena of Zurzach, mostly just called ''Saint Verena'' (c. 260 – c. 320) is an early Christian consecrated virgin and hermit. She is especially venerated in Switzerland, where her cult is attested in Bad Zurzach, the reported place of he ...
and Jean-Jacques Kirkyacharian. They didn't take blood analyses but during their trip they interviewed health personnel, children and their parents. They also took detailed notes of the symptoms. They wrote that some doctors had noticed a smell from the students which was similar to "vinegar
Vinegar is an aqueous solution of acetic acid and trace compounds that may include flavorings. Vinegar typically contains 5–8% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to et ...
". According to them, it was possible that the disease was caused from poisoning which might have been in the form of organic phosphates (nerve gas). The suspicion of nerve gases
Nerve agents, sometimes also called nerve gases, are a class of organic chemistry, organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (ACh ...
was reinforced in February 1992 when Aubin Heyndrickx gave a press statement in which he claimed that he had studied all reports and analysis of blood and urine and that he concluded that an organic chemical nerve gas had been used such as Sarin and Tabun, both listed as warfare agents.
* 1994: Matsumoto incident
The Matsumoto sarin attack was an attempted assassination perpetrated by members of the Aum Shinrikyo doomsday cult in Matsumoto, Nagano Prefecture, Japan on the night of June 27, 1994. Eight people were killed
; the Japanese religious sect Aum Shinrikyo
, formerly , is a Japanese doomsday cult founded by Shoko Asahara in 1987. It carried out the deadly Tokyo subway sarin attack in 1995 and was found to have been responsible for the Matsumoto sarin attack the previous year.
The group says tha ...
released an impure form of sarin in Matsumoto, Nagano
is a city located in Nagano Prefecture, Japan. Matsumoto is designated as a core city since 1 April 2021. , the city had a population of 239,466 in 105,207 households and a population density of 240 persons per km2. The total area of the city ...
, killing eight people and harming over 500. The Australian sheep station Banjawarn was a testing ground.
* 1995: Tokyo subway sarin attack
The was an act of domestic terrorism perpetrated on 20 March 1995, in Tokyo, Japan, by members of the cult movement Aum Shinrikyo. In five coordinated attacks, the perpetrators released sarin on three lines of the Tokyo Metro (then ''Teito Rapi ...
; the Aum Shinrikyo
, formerly , is a Japanese doomsday cult founded by Shoko Asahara in 1987. It carried out the deadly Tokyo subway sarin attack in 1995 and was found to have been responsible for the Matsumoto sarin attack the previous year.
The group says tha ...
sect released an impure form of sarin in the Tokyo Metro
The is a major rapid transit system in Tokyo, Japan, operated by the Tokyo Metro Co. With an average daily ridership of 6.84 million passengers, the Tokyo Metro is the larger of the two subway operators in the city; the other being the Toei ...
. Twelve people died, and over 6,200 people received injuries.
* 2002: Pro- Chechen militant Ibn al-Khattab may have been assassinated with sarin by the Russian government.
* May 2004: Iraqi insurgents detonated a 155 mm shell containing binary precursors for sarin near a U.S. convoy in Iraq
Iraq,; ku, عێراق, translit=Êraq officially the Republic of Iraq, '; ku, کۆماری عێراق, translit=Komarî Êraq is a country in Western Asia. It is bordered by Turkey to Iraq–Turkey border, the north, Iran to Iran–Iraq ...
. The shell was designed to mix the chemicals as it spun during flight. The detonated shell released only a small amount of sarin gas, either because the explosion failed to mix the binary agents properly or because the chemicals inside the shell had degraded with age. Two United States soldiers were treated after displaying the early symptoms of exposure to sarin.
* March 2013: Khan al-Assal chemical attack
The Khan al-Assal chemical attack was a chemical attack in Khan al-Assal, Aleppo, Syria on 19 March 2013, which according to the Syrian Observatory for Human Rights resulted in at least 26 fatalities including 16 government soldiers and 10 civi ...
; Sarin was used in an attack on a town west of Aleppo
)), is an adjective which means "white-colored mixed with black".
, motto =
, image_map =
, mapsize =
, map_caption =
, image_map1 =
...
city in Syria
Syria ( ar, سُورِيَا or سُورِيَة, translit=Sūriyā), officially the Syrian Arab Republic ( ar, الجمهورية العربية السورية, al-Jumhūrīyah al-ʻArabīyah as-Sūrīyah), is a Western Asian country loc ...
, killing 28 and wounding 124.
* August 2013: Ghouta chemical attack
The Ghouta chemical attack, was a Chemical warfare, chemical attack carried out by the forces of Syrian President Bashar al-Assad, in the early hours of 21 August 2013 in Ghouta, Syria during the Syrian civil war. Two Syrian opposition, oppos ...
; Sarin was used in multiple simultaneous attacks in the Ghouta
Ghouta ( ar, غُوطَةُ دِمَشْقَ / ALA-LC: ''Ḡūṭat Dimašq'') is a countryside and suburban area in southwestern Syria that surrounds the city of Damascus along its eastern and southern rim.
Name
Ghouta is the Arabic term (''gh ...
region of the Rif Dimashq
Rif Dimashq Governorate ( ar, محافظة ريف دمشق, ', literally, the "Governorate of the Countryside of Damascus", Damascus Suburb) is one of the fourteen governorates (provinces) of Syria. It is situated in the southwestern part of the c ...
Governorate of Syria during the Syrian Civil War. Varying sources gave a death toll of 322 to 1,729.
* April 2017: Khan Shaykhun chemical attack
The Khan Shaykhun chemical attack took place on 4 April 2017 on the town of Khan Shaykhun in the Idlib Governorate of Syria. The town was reported to have been struck by an airstrike by government forces followed by massive civilian chemical ...
; Sarin gas was released in rebel-held Idlib Province in Syria by the Syrian Air Force
)
, mascot =
, anniversaries = 16 October
, equipment =
, equipment_label =
, battles = * 1948 Arab-Israeli War
* Six-Day War
* Yom Kippur War
...
during an airstrike
An airstrike, air strike or air raid is an offensive operation carried out by aircraft. Air strikes are delivered from aircraft such as blimps, balloons, fighters, heavy bombers, ground attack aircraft, attack helicopters and drones. The offic ...
.
* April 2018: Douma chemical attack
On 7 April 2018, a chemical warfare attack was carried out by forces of the government of Bashar al-Assad in the Syrian city of Douma. Medics and witnesses reported that it caused the deaths of between 40 and 50 people and injuries to possi ...
victims reported to have symptoms consistent with exposure to sarin and other agents. On 6 July 2018, the Fact-Finding Mission (FFM) of the OPCW published their interim report. The report stated that, "The results show that no organophosphorous arin
The American Registry for Internet Numbers (ARIN) is the regional Internet registry for Canada, the United States, and many Caribbean and North Atlantic islands. ARIN manages the distribution of Internet number resources, including IPv4 and IPv ...
nerve agents or their degradation products were detected in the environmental samples or in the plasma samples taken from alleged casualties". The chemical agent used in the attack was later identified as elemental chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
.
See also
*Chlorosarin
Chlorosarin is a chemical precursor used in the final step of one method for the production of the nerve agent sarin. Also known as ''O''-isopropyl methylphosphonochloridate and isopropyl methylphosphonic chloride, it has a molecular weight of ...
* Ethylsarin
Ethylsarin (GE), also known as EA-1209, TL-1620 or T-2109, is an organophosphate nerve agent of the G-series. It is the ethylphosphonofluoridate analog of sarin
Sarin (NATO designation GB G-series, "B"">Nerve_agent#G-series.html" ;"title= ...
* Thiosarin
* Gulf War syndrome
Gulf War syndrome or Gulf War illness is a chronic and multi-symptomatic disorder affecting military veterans of both sides of the 1990–1991 Persian Gulf War. A wide range of acute and chronic symptoms have been linked to it, including fatigue ...
References
External links
Material Safety Data Sheet
CDC Sarin fact sheet
{{Phosphorus compounds Acetylcholinesterase inhibitors Chemical weapons of the United States Cold War weapons of the Soviet Union G-series nerve agents German chemical weapons program German inventions of the Nazi period Isopropyl esters Methylphosphonofluoridates Soviet chemical weapons program Substances discovered in the 1930s Toxicology United Kingdom chemical weapons program