Sulfur chlorides on:
[Wikipedia]
[Google]
[Amazon]
Sulfur (or sulphur in
here
The greatest commercial use of the element is the production of

 Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is more of a deep yellow than the S8.
Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is more of a deep yellow than the S8.
 32S is created inside massive stars, at a depth where the temperature exceeds 2.5×109 K, by the
32S is created inside massive stars, at a depth where the temperature exceeds 2.5×109 K, by the
 This reaction highlights a distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains).
This reaction highlights a distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains).
File:Allicin skeletal.svg,
Some of the main classes of sulfur-containing organic compounds include the following:
*
 Being abundantly available in native form, sulfur was known in ancient times and is referred to in the
Being abundantly available in native form, sulfur was known in ancient times and is referred to in the
British English
British English (BrE, en-GB, or BE) is, according to Oxford Dictionaries, "English as used in Great Britain, as distinct from that used elsewhere". More narrowly, it can refer specifically to the English language in England, or, more broadl ...
) is a chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
S and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
16. It is abundant, multivalent and nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differentl ...
lic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
line solid at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
.
Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native
Native may refer to:
People
* Jus soli, citizenship by right of birth
* Indigenous peoples, peoples with a set of specific rights based on their historical ties to a particular territory
** Native Americans (disambiguation)
In arts and entert ...
form, sulfur on Earth usually occurs as sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
and sulfate minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary mine ...
. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India
According to consensus in modern genetics, anatomically modern humans first arrived on the Indian subcontinent from Africa between 73,000 and 55,000 years ago. Quote: "Y-Chromosome and Mt-DNA data support the colonization of South Asia by ...
, ancient Greece
Ancient Greece ( el, Ἑλλάς, Hellás) was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12th–9th centuries BC to the end of classical antiquity ( AD 600), that comprised a loose collection of cu ...
, China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's List of countries and dependencies by population, most populous country, with a Population of China, population exceeding 1.4 billion, slig ...
, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
and petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
.. Downloahere
The greatest commercial use of the element is the production of
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
for sulfate and phosphate fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
s, and other chemical processes. Sulfur is used in match
A match is a tool for starting a fire. Typically, matches are made of small wooden sticks or stiff paper. One end is coated with a material that can be ignited by friction generated by striking the match against a suitable surface. Wooden mat ...
es, insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed t ...
s, and fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality ...
s. Many sulfur compounds are odoriferous, and the smells of odorized natural gas, skunk scent, grapefruit, and garlic are due to organosulfur
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur ...
compounds. Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
gives the characteristic odor to rotting eggs and other biological processes.
Sulfur is an essential element
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight ( oxygen, hydrogen, ca ...
for all life, but almost always in the form of organosulfur compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulf ...
or metal sulfides. Amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s (two proteinogenic
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino ...
: cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
and methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ...
, and many other non-coded: cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mec ...
, taurine
Taurine (), or 2-aminoethanesulfonic acid, is an organic compound that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight. I ...
, etc.) and two vitamins (biotin
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', bo ...
and thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, that cannot be made in the body. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thi ...
) are organosulfur compounds crucial for life. Many cofactors
Cofactor may also refer to:
* Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed
* A domain parameter in elliptic curve cryptography, defined as the ratio between the order ...
also contain sulfur, including glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
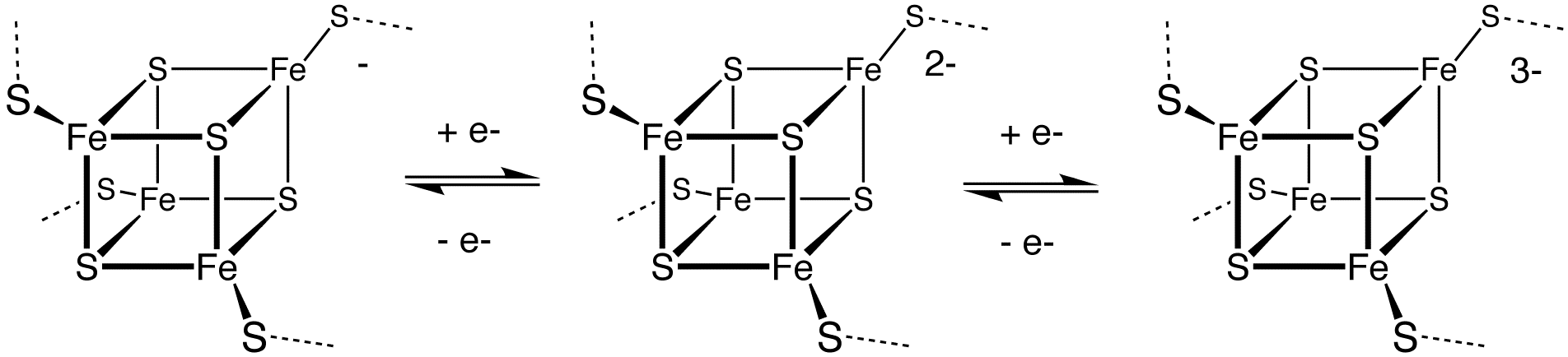
, and iron–sulfur protein
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur c ...
s. Disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s, S–S bonds, confer mechanical strength and insolubility of the (among others) protein keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up Scale (anatomy), scales, hair, Nail ...
, found in outer skin, hair, and feathers. Sulfur is one of the core chemical elements needed for biochemical
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology an ...
functioning and is an elemental macronutrient
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excr ...
for all living organisms.
Characteristics

Physical properties
Sulfur forms several polyatomic molecules. The best-known allotrope isoctasulfur
Octasulfur is an inorganic substance with the chemical formula . It is an odourless and tasteless yellow solid, and is a major industrial chemical. It is the most common allotrope of sulfur and occurs widely in nature.Steudel, R., "Homocyclic Sul ...
, cyclo-S8. The point group
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every ...
of cyclo-S8 is D4d and its dipole moment is 0 D. Octasulfur is a soft, bright-yellow solid that is odorless, but impure samples have an odor similar to that of match
A match is a tool for starting a fire. Typically, matches are made of small wooden sticks or stiff paper. One end is coated with a material that can be ignited by friction generated by striking the match against a suitable surface. Wooden mat ...
es. It melts at , boils at and sublimes more or less between and . At , below its melting temperature, cyclo-octasulfur changes from α-octasulfur to the β- polymorph. The structure of the S8 ring is virtually unchanged by this phase change, which affects the intermolecular interactions. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the int ...
due to the formation of polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s. At higher temperatures, the viscosity decreases as depolymerization occurs. Molten sulfur assumes a dark red color above . The density of sulfur is about 2 g/cm3, depending on the allotrope; all of the stable allotropes are excellent electrical insulators.
Sulfur is insoluble in water but soluble in carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
and, to a lesser extent, in other nonpolar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
organic solvents, such as benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
.
Chemical properties
Under normal conditions, sulfurhydrolyzes
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
very slowly to mainly form hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
:
: + 4 → 3 +
The reaction involves adsorption of protons onto clusters, followed by disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can ...
into the reaction products.
The second, fourth and sixth ionization energies
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecul ...
of sulfur are 2252 kJ/mol−1, 4556 kJ/mol−1 and 8495.8 kJ/mol−1,respectively. A composition of products of sulfur's reactions with oxidants (and its oxidation state) depends on that whether releasing out of a reaction energy overcomes these thresholds. Applying catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
and / or supply of outer energy may vary sulfur's oxidation state and a composition of reaction products. While reaction between sulfur and oxygen at normal conditions gives sulfur dioxide (oxidation state +4), formation of sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an ind ...
(oxidation state +6) requires temperature 400 – 600 °C and presence of a catalyst.
In reactions with elements electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of which less than sulfur's, it comes as an oxidant, and forms sulfides with oxidation state –2.
Sulfur reacts with nearly all other elements with the exception of the noble gases, even with the notoriously unreactive metal iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density o ...
(yielding iridium disulfide).
Some of those reactions need elevated temperatures.
Allotropes
 Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is more of a deep yellow than the S8.
Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is more of a deep yellow than the S8. HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to p ...
analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but with S7 and small amounts of S6. Larger rings have been prepared, including S12 and S18.
Amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language, Gr ...
or "plastic" sulfur is produced by rapid cooling of molten sulfur—for example, by pouring it into cold water. X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
studies show that the amorphous form may have a helical
Helical may refer to:
* Helix, the mathematical concept for the shape
* Helical engine, a proposed spacecraft propulsion drive
* Helical spring, a coilspring
* Helical plc, a British property company, once a maker of steel bar stock
* Helicoil
A t ...
structure with eight atoms per turn. The long coiled polymeric molecules make the brownish substance elastic
Elastic is a word often used to describe or identify certain types of elastomer, elastic used in garments or stretchable fabrics.
Elastic may also refer to:
Alternative name
* Rubber band, ring-shaped band of rubber used to hold objects togethe ...
, and in bulk this form has the feel of crude rubber. This form is metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
at room temperature and gradually reverts to crystalline molecular allotrope, which is no longer elastic. This process happens within a matter of hours to days, but can be rapidly catalyzed.
Isotopes
Sulfur has 23 knownisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s, four of which are stable: 32S (), 33S (), 34S (), and 36S (). Other than 35S, with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 87 days and formed in cosmic ray spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly ener ...
of 40 Ar, the radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
isotopes of sulfur have half-lives less than 3 hours.
When sulfide mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, th ...
s are precipitated, isotopic equilibration among solids and liquid may cause small differences in the δ34S values of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. The δ13C and δ34S of coexisting carbonate minerals and sulfides can be used to determine the pH and oxygen fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas whic ...
of the ore-bearing fluid during ore formation.
In most forest
A forest is an area of land dominated by trees. Hundreds of definitions of forest are used throughout the world, incorporating factors such as tree density, tree height, land use, legal standing, and ecological function. The United Nations' ...
ecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites contribute some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in hydrologic
Hydrology () is the scientific study of the movement, distribution, and management of water on Earth and other planets, including the water cycle, water resources, and environmental watershed sustainability. A practitioner of hydrology is calle ...
studies. Differences in the natural abundances can be used in systems where there is sufficient variation in the 34S of ecosystem components. Rocky Mountain
The Rocky Mountains, also known as the Rockies, are a major mountain range and the largest mountain system in North America. The Rocky Mountains stretch in straight-line distance from the northernmost part of western Canada, to New Mexico in ...
lakes thought to be dominated by atmospheric sources of sulfate have been found to have characteristic 34S values from lakes believed to be dominated by watershed sources of sulfate.
Natural occurrence
 32S is created inside massive stars, at a depth where the temperature exceeds 2.5×109 K, by the
32S is created inside massive stars, at a depth where the temperature exceeds 2.5×109 K, by the fusion
Fusion, or synthesis, is the process of combining two or more distinct entities into a new whole.
Fusion may also refer to:
Science and technology Physics
*Nuclear fusion, multiple atomic nuclei combining to form one or more different atomic nucl ...
of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the alpha process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process.
The triple-alpha process consumes only helium, a ...
that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or moon. When the original object ...
s. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. It is normally present as troilite
Troilite is a rare iron sulfide mineral with the simple formula of FeS. It is the iron-rich endmember of the pyrrhotite group. Pyrrhotite has the formula Fe(1-x)S (x = 0 to 0.2) which is iron deficient. As troilite lacks the iron deficiency whic ...
(FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds. The distinctive colors of Jupiter
Jupiter is the fifth planet from the Sun and the largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but slightly less than one-thousand ...
's volcanic
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plat ...
moon Io are attributed to various forms of molten, solid, and gaseous sulfur.
It is the fifth most common element by mass in the Earth. Elemental sulfur can be found near hot spring
A hot spring, hydrothermal spring, or geothermal spring is a spring produced by the emergence of geothermally heated groundwater onto the surface of the Earth. The groundwater is heated either by shallow bodies of magma (molten rock) or by circ ...
s and volcanic
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plat ...
regions in many parts of the world, especially along the Pacific Ring of Fire
The Ring of Fire (also known as the Pacific Ring of Fire, the Rim of Fire, the Girdle of Fire or the Circum-Pacific belt) is a region around much of the rim of the Pacific Ocean where many volcanic eruptions and earthquakes occur. The Ring ...
; such volcanic deposits are currently mined in Indonesia, Chile, and Japan. These deposits are polycrystalline, with the largest documented single crystal measuring 22×16×11 cm. Historically, Sicily
(man) it, Siciliana (woman)
, population_note =
, population_blank1_title =
, population_blank1 =
, demographics_type1 = Ethnicity
, demographics1_footnotes =
, demographi ...
was a major source of sulfur in the Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
. Lakes of molten sulfur up to ~200 m in diameter have been found on the sea floor, associated with submarine volcano
Submarine volcanoes are underwater vents or fissures in the Earth's surface from which magma can erupt. Many submarine volcanoes are located near areas of tectonic plate formation, known as mid-ocean ridges. The volcanoes at mid-ocean ri ...
es, at depths where the boiling point of water is higher than the melting point of sulfur.C. E. J. de Ronde, W. W. Chadwick Jr, R. G. Ditchburn, R. W. Embley, V. Tunnicliffe, E. T. Baker. S. L. Walker. V. L. Ferrini, and S. M. Merle (2015): "Molten Sulfur Lakes of Intraoceanic Arc Volcanoes". Chapter of ''Volcanic Lakes'' (Springer), pages 261-288.
Native sulfur is synthesised by anaerobic bacteria
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenat ...
acting on sulfate minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary mine ...
such as gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywa ...
in salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered usi ...
s. Significant deposits in salt domes occur along the coast of the Gulf of Mexico
The Gulf of Mexico ( es, Golfo de México) is an ocean basin and a marginal sea of the Atlantic Ocean, largely surrounded by the North American continent. It is bounded on the northeast, north and northwest by the Gulf Coast of the United ...
, and in evaporite
An evaporite () is a water- soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocean ...
s in eastern Europe and western Asia. Native sulfur may be produced by geological processes alone. Fossil-based sulfur deposits from salt domes were once the basis for commercial production in the United States, Russia, Turkmenistan, and Ukraine. Currently, commercial production is still carried out in the Osiek mine in Poland. Such sources are now of secondary commercial importance, and most are no longer worked.
Common naturally occurring sulfur compounds include the sulfide minerals
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, ...
, such as pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Iron, FeSulfur, S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic Luster (mineralogy), lust ...
(iron sulfide), cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the bri ...
(mercury sulfide), galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cr ...
(lead sulfide), sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-V ...
(zinc sulfide), and stibnite
Stibnite, sometimes called antimonite, is a sulfide mineral with the formula Sb2 S3. This soft grey material crystallizes in an orthorhombic space group. It is the most important source for the metalloid antimony. The name is derived from the ...
(antimony sulfide); and the sulfate minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary mine ...
, such as gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywa ...
(calcium sulfate), alunite
Alunite is a hydroxylated aluminium potassium sulfate mineral, formula K Al3( S O4)2(O H)6. It was first observed in the 15th century at Tolfa, near Rome, where it was mined for the manufacture of alum. First called ''aluminilite'' by J.C. D ...
(potassium aluminium sulfate), and barite
Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate ( Ba S O4). Baryte is generally white or colorless, and is the main source of the element barium. The ''baryte group'' consists of baryte, celestine (strontium sulfate), ...
(barium sulfate). On Earth, just as upon Jupiter's moon Io, elemental sulfur occurs naturally in volcanic emissions, including emissions from hydrothermal vent
A hydrothermal vent is a fissure on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspo ...
s.
The main industrial source of sulfur is now petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
and natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
.
Compounds
Commonoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
es.
Electron transfer reactions
Sulfur polycations, S82+, S42+ and S162+ are produced when sulfur is reacted with oxidising agents in a strongly acidic solution. The colored solutions produced by dissolving sulfur inoleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid). Ol ...
were first reported as early as 1804 by C.F. Bucholz, but the cause of the color and the structure of the polycations involved was only determined in the late 1960s. S82+ is deep blue, S42+ is yellow and S162+ is red.
Reduction of sulfur gives various polysulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anions, which have the general formu ...
s with the formula Sx2-, many of which have been obtained crystalline form. Illustrative is the production of sodium tetrasulfide:
:
Some of these dianions dissociate to give radical anion
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of ...
s, such as S3− gives the blue color of the rock lapis lazuli
Lapis lazuli (; ), or lapis for short, is a deep-blue metamorphic rock used as a semi-precious stone that has been prized since antiquity for its intense color.
As early as the 7th millennium BC, lapis lazuli was mined in the Sar-i Sang mine ...
.
 This reaction highlights a distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains).
This reaction highlights a distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains). Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
of these polysulfide anions produces the polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. Compounds containing 2 – 8 concatenated ...
s, H2Sx where x= 2, 3, and 4. Ultimately, reduction of sulfur produces sulfide salts:
:16 Na + S8 → 8 Na2S
The interconversion of these species is exploited in the sodium–sulfur battery.
Hydrogenation
Treatment of sulfur with hydrogen giveshydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
. When dissolved in water, hydrogen sulfide is mildly acidic:Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.), Oxford:Butterworth-Heinemann. .
:H2S HS− + H+
Hydrogen sulfide gas and the hydrosulfide anion are extremely toxic to mammals, due to their inhibition of the oxygen-carrying capacity of hemoglobin and certain cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of ...
s in a manner analogous to cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
and azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant appli ...
(see below, under ''precautions'').
Combustion
The two principal sulfur oxides are obtained by burning sulfur: :S + O2 → SO2 (sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
)
:2 SO2 + O2 → 2 SO3 (sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an ind ...
)
Many other sulfur oxides are observed including the sulfur-rich oxides include sulfur monoxide, disulfur monoxide, disulfur dioxides, and higher oxides containing peroxo groups.
Halogenation
Sulfur reacts with fluorine to give the highly reactive sulfur tetrafluoride and the highly inertSulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non- flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attach ...
. Whereas fluorine gives S(IV) and S(VI) compounds, chlorine gives S(II) and S(I) derivatives. Thus, sulfur dichloride
Sulfur dichloride is the chemical compound with the formula . This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance ...
, disulfur dichloride
Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2.
Some alternative names for this compound are ''sulfur monochloride'' (the name implied by its empirical formula, SCl), ''disulphur dichloride'' (Britis ...
, and higher chlorosulfanes arise from the chlorination of sulfur. Sulfuryl chloride
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis.
Sulfuryl chloride is ...
and chlorosulfuric acid
Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid which is hygroscopic an ...
are derivatives of sulfuric acid; thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
(SOCl2) is a common reagent in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
.
Pseudohalides
Sulfur oxidizescyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
and sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are w ...
to give thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyr ...
and thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e ...
, respectively.
Metal sulfides
Sulfur reacts with many metals. Electropositive metals give polysulfide salts. Copper, zinc, silver are attacked by sulfur, seetarnishing
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
. Although many metal sulfides are known, most are prepared by high temperature reactions of the elements.
Organic compounds
Allicin
Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, th ...
, a chemical compound in garlic
File:L-Cystein - L-Cysteine.svg , (''R'')-cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
, an amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
containing a thiol group
File:Methionin - Methionine.svg, Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ...
, an amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
containing a thioether
File:Diphenyl disulfide.svg, Diphenyl disulfide
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiop ...
, a representative disulfide
File:Perfluorooctanesulfonic acid structure.svg, Perfluorooctanesulfonic acid
Perfluorooctanesulfonic acid (PFOS) (conjugate base perfluorooctanesulfonate) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group and thus a perfluorosulfonic acid. It is an anthropogenic (man-ma ...
, a surfactant
File:Dibenzothiophen - Dibenzothiophene.svg, Dibenzothiophene
Dibenzothiophene (DBT, diphenylene sulfide) is the organosulfur compound consisting of two benzene rings fused to a central thiophene ring. It is a colourless solid that is chemically somewhat similar to anthracene. This tricyclic heterocycle, ...
, a component of crude oil
File:Penicillin core.svg, Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from '' Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum usin ...
, an antibiotic where "R" is the variable group
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
s or mercaptans (so called because they capture mercury as chelators) are the sulfur analogs of alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s; treatment of thiols with base gives thiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
ions.
* Thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...
s are the sulfur analogs of ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again ...
s.
* Sulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a "cation") featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula . Together wi ...
ions have three groups attached to a cationic sulfur center. Dimethylsulfoniopropionate
Dimethylsulfoniopropionate (DMSP), is an organosulfur compound with the formula (CH3)2S+CH2CH2COO−. This zwitterionic metabolite can be found in marine phytoplankton, seaweeds, and some species of terrestrial and aquatic vascular plants ...
(DMSP) is one such compound, important in the marine organic sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a con ...
.
* Sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s and sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s are thioethers with one and two oxygen atoms attached to the sulfur atom, respectively. The simplest sulfoxide, dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds a ...
, is a common solvent; a common sulfone is sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solve ...
.
* Sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is k ...
s are used in many detergents.
Compounds with carbon–sulfur multiple bonds are uncommon, an exception being carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
, a volatile colorless liquid that is structurally similar to carbon dioxide. It is used as a reagent to make the polymer rayon
Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. It is also called viscose. Many types and grades of viscose ...
and many organosulfur compounds. Unlike carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
, carbon monosulfide
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interst ...
is stable only as an extremely dilute gas, found between solar systems.
Organosulfur compounds are responsible for some of the unpleasant odors of decaying organic matter. They are widely known as the odorant
An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently vol ...
in domestic natural gas, garlic odor, and skunk spray. Not all organic sulfur compounds smell unpleasant at all concentrations: the sulfur-containing monoterpenoid
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen funct ...
( grapefruit mercaptan) in small concentrations is the characteristic scent of grapefruit, but has a generic thiol odor at larger concentrations. Sulfur mustard
Mustard gas or sulfur mustard is a chemical compound belonging to a family of cytotoxic and blister agents known as mustard agents. The name ''mustard gas'' is technically incorrect: the substance, when dispersed, is often not actually a gas, ...
, a potent vesicant
A blister agent (or vesicant), is a chemical compound that causes severe skin, eye and mucosal pain and irritation. They are named for their ability to cause severe chemical burns, resulting in painful water blisters on the bodies of those affec ...
, was used in World War I as a disabling agent.
Sulfur–sulfur bonds are a structural component used to stiffen rubber, similar to the disulfide bridges that rigidify proteins (see biological below). In the most common type of industrial "curing" or hardening and strengthening of natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
, elemental sulfur is heated with the rubber to the point that chemical reactions form disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
bridges between isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals ...
units of the polymer. This process, patented in 1843, made rubber a major industrial product, especially in automobile tires. Because of the heat and sulfur, the process was named vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
, after the Roman god of the forge and volcanism
Volcanism, vulcanism or volcanicity is the phenomenon of eruption of molten rock (magma) onto the surface of the Earth or a solid-surface planet or moon, where lava, pyroclastics, and volcanic gases erupt through a break in the surface called a ...
.
History
Antiquity
 Being abundantly available in native form, sulfur was known in ancient times and is referred to in the
Being abundantly available in native form, sulfur was known in ancient times and is referred to in the Torah
The Torah (; hbo, ''Tōrā'', "Instruction", "Teaching" or "Law") is the compilation of the first five books of the Hebrew Bible, namely the books of Genesis, Exodus, Leviticus, Numbers and Deuteronomy. In that sense, Torah means the ...
(Genesis
Genesis may refer to:
Bible
* Book of Genesis, the first book of the biblical scriptures of both Judaism and Christianity, describing the creation of the Earth and of mankind
* Genesis creation narrative, the first several chapters of the Book of ...
). English translations of the Christian Bible commonly referred to burning sulfur as "brimstone", giving rise to the term " fire-and-brimstone" sermon
A sermon is a religious discourse or oration by a preacher, usually a member of clergy. Sermons address a scriptural, theological, or moral topic, usually expounding on a type of belief, law, or behavior within both past and present contexts. ...
s, in which listeners are reminded of the fate of eternal damnation
Damnation (from Latin '' damnatio'') is the concept of divine punishment and torment in an afterlife for actions that were committed, or in some cases, not committed on Earth.
In Ancient Egyptian religious tradition, citizens would recite th ...
that await the unbelieving and unrepentant. It is from this part of the Bible that Hell
In religion and folklore, hell is a location in the afterlife in which evil souls are subjected to punitive suffering, most often through torture, as eternal punishment after death. Religions with a linear divine history often depict hell ...
is implied to "smell of sulfur" (likely due to its association with volcanic activity). According to the Ebers Papyrus, a sulfur ointment was used in ancient Egypt
Egypt ( ar, مصر , ), officially the Arab Republic of Egypt, is a List of transcontinental countries, transcontinental country spanning the North Africa, northeast corner of Africa and Western Asia, southwest corner of Asia via a land bridg ...
to treat granular eyelids. Sulfur was used for fumigation in preclassical Greece
Greece,, or , romanized: ', officially the Hellenic Republic, is a country in Southeast Europe. It is situated on the southern tip of the Balkans, and is located at the crossroads of Europe, Asia, and Africa. Greece shares land borders wi ...
; this is mentioned in the ''Odyssey
The ''Odyssey'' (; grc, Ὀδύσσεια, Odýsseia, ) is one of two major ancient Greek epic poems attributed to Homer. It is one of the oldest extant works of literature still widely read by modern audiences. As with the '' Iliad'', ...
''. Pliny the Elder
Gaius Plinius Secundus (AD 23/2479), called Pliny the Elder (), was a Roman author, naturalist and natural philosopher, and naval and army commander of the early Roman Empire, and a friend of the emperor Vespasian. He wrote the encyclopedic ' ...
discusses sulfur in book 35 of his '' Natural History'', saying that its best-known source is the island of Melos
Milos or Melos (; el, label=Modern Greek, Μήλος, Mílos, ; grc, Μῆλος, Mêlos) is a volcanic Greek island in the Aegean Sea, just north of the Sea of Crete. Milos is the southwesternmost island in the Cyclades group.
The '' Venus ...
. He mentions its use for fumigation, medicine, and bleaching cloth.
A natural form of sulfur known as () was known in China since the 6th century BC and found in Hanzhong
Hanzhong (; abbreviation: Han) is a prefecture-level city in the southwest of Shaanxi province, China, bordering the provinces of Sichuan to the south and Gansu to the west.
The founder of the Han dynasty, Liu Bang, was once enfeoffed as ...
. By the 3rd century, the Chinese had discovered that sulfur could be extracted from pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Iron, FeSulfur, S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic Luster (mineralogy), lust ...
. Chinese Daoists were interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found in traditional Chinese medicine
Traditional Chinese medicine (TCM) is an alternative medical practice drawn from traditional medicine in China. It has been described as "fraught with pseudoscience", with the majority of its treatments having no logical mechanism of acti ...
. A Song dynasty
The Song dynasty (; ; 960–1279) was an imperial dynasty of China that began in 960 and lasted until 1279. The dynasty was founded by Emperor Taizu of Song following his usurpation of the throne of the Later Zhou. The Song conquered the res ...
military treatise of 1044 AD described various formulas for Chinese black powder
Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, carbon (in the form of charcoal) and potassium nitrate (saltpeter). T ...
, which is a mixture of potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and ...
(), charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ...
, and sulfur. It remains an ingredient of black gunpowder.
Indian alchemists, practitioners of the "science of chemicals" ( sa, रसशास्त्र, rasaśāstra), wrote extensively about the use of sulfur in alchemical operations with mercury, from the eighth century AD onwards. In the tradition, sulfur is called "the smelly" (, ).
Early Europe
Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...
an alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim ...
gave sulfur a unique alchemical symbol
Alchemical symbols, originally devised as part of alchemy, were used to denote some elements and some compounds until the 18th century. Although notation like this was mostly standardized, style and symbol varied between alchemists, so this pag ...
, a triangle atop a cross (🜍). (This is sometimes confused with the astronomical crossed-spear symbol ⚴ for 2 Pallas
Pallas ( minor-planet designation: 2 Pallas) is the second asteroid to have been discovered, after Ceres. It is believed to have a mineral composition similar to carbonaceous chondrite meteorites, like Ceres, though significantly less hy ...
.) The variation known as brimstone has a symbol combining a two-barred cross
A two-barred cross is similar to a Latin cross but with an extra bar added. The lengths and placement of the bars (or "arms") vary, and most of the variations are interchangeably called the cross of Lorraine, the patriarchal cross, the Orthodox ...
atop a lemniscate
In algebraic geometry, a lemniscate is any of several figure-eight or -shaped curves. The word comes from the Latin "''lēmniscātus''" meaning "decorated with ribbons", from the Greek λημνίσκος meaning "ribbons",. or which alternative ...
(🜏). In traditional skin treatment, elemental sulfur was used (mainly in creams) to alleviate such conditions as scabies
Scabies (; also sometimes known as the seven-year itch) is a contagious skin infestation by the mite ''Sarcoptes scabiei''. The most common symptoms are severe itchiness and a pimple-like rash. Occasionally, tiny burrows may appear on the ski ...
, ringworm
Dermatophytosis, also known as ringworm, is a fungal infection of the skin. Typically it results in a red, itchy, scaly, circular rash. Hair loss may occur in the area affected. Symptoms begin four to fourteen days after exposure. Multiple ar ...
, psoriasis
Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by raised areas of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small, localized patches to comple ...
, eczema
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved c ...
, and acne
Acne, also known as ''acne vulgaris'', is a long-term skin condition that occurs when dead skin cells and oil from the skin clog hair follicles. Typical features of the condition include blackheads or whiteheads, pimples, oily skin, and ...
. The mechanism of action is unknown—though elemental sulfur does oxidize slowly to sulfurous acid, which is (through the action of sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are w ...
) a mild reducing and antibacterial agent.
Modern times
Sulfur appears in a column of fixed (non-acidic)alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
in a chemical table of 1718. Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794),
CNRS (
 Sulfur may be found by itself and historically was usually obtained in this form;
Sulfur may be found by itself and historically was usually obtained in this form;  Elemental sulfur was extracted from
Elemental sulfur was extracted from  Owing to the high sulfur content of the Athabasca Oil Sands, stockpiles of elemental sulfur from this process now exist throughout Alberta, Canada. Another way of storing sulfur is as a binder (material), binder for concrete, the resulting product having many desirable properties (see sulfur concrete).
Sulfur is still mined from surface deposits in poorer nations with volcanoes, such as Indonesia, and worker conditions have not improved much since Booker T. Washington's days.
The world production of sulfur in 2011 amounted to 69 million tonnes (Mt), with more than 15 countries contributing more than 1 Mt each. Countries producing more than 5 Mt are China (9.6), the United States (8.8), Canada (7.1) and Russia (7.1). Production has been slowly increasing from 1900 to 2010; the price was unstable in the 1980s and around 2010.
Owing to the high sulfur content of the Athabasca Oil Sands, stockpiles of elemental sulfur from this process now exist throughout Alberta, Canada. Another way of storing sulfur is as a binder (material), binder for concrete, the resulting product having many desirable properties (see sulfur concrete).
Sulfur is still mined from surface deposits in poorer nations with volcanoes, such as Indonesia, and worker conditions have not improved much since Booker T. Washington's days.
The world production of sulfur in 2011 amounted to 69 million tonnes (Mt), with more than 15 countries contributing more than 1 Mt each. Countries producing more than 5 Mt are China (9.6), the United States (8.8), Canada (7.1) and Russia (7.1). Production has been slowly increasing from 1900 to 2010; the price was unstable in the 1980s and around 2010.
 In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
 Elemental sulfur is one of the oldest fungicides and pesticides. "Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organic agriculture, organically farmed apple production against the main disease apple scab under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or Aerial application, from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscibility, miscible. It has similar applications and is used as a
Elemental sulfur is one of the oldest fungicides and pesticides. "Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organic agriculture, organically farmed apple production against the main disease apple scab under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or Aerial application, from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscibility, miscible. It has similar applications and is used as a
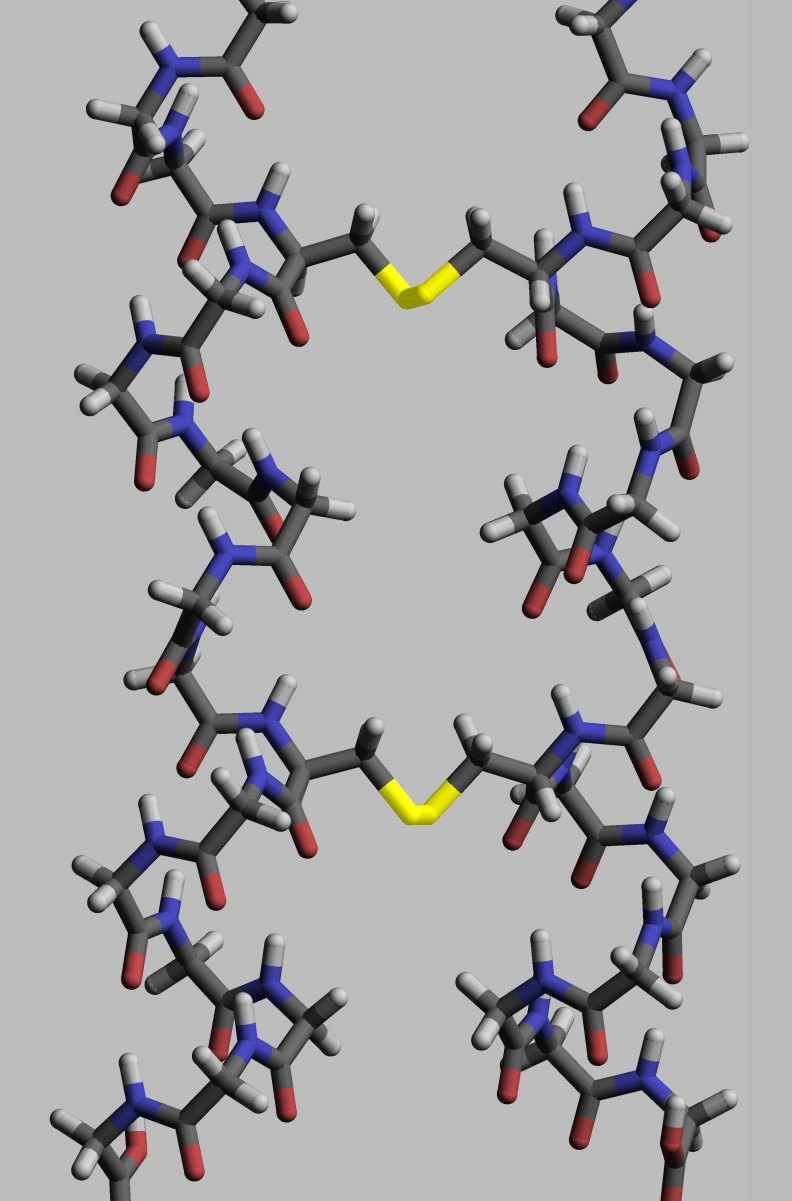 Proteins, to execute their Protein#Cellular functions, biological function, need to have specific space geometry. Formation of this geometry is performed in a process called protein folding, and is provided by intra- and inter-molecular bonds. The process has several stages. While at premier stages a polypeptide chain folds due to hydrogen bonds, at later stages folding is provided (apart from hydrogen bonds) by covalent bonds between two sulfur atoms of two cysteine residues (so called disulfide bridges) at different places of a chain (tertriary protein structure) as well as between two cysteine residues in two separated protein subunits (quaternary protein structure). Both structures easily may be seen in insulin. As the bond energy of a covalent disulfide bridge is higher than the energy of a Coordinate covalent bond, coordinate bond or hydrophylic either hydrophobic interaction, the higher disulfide bridges content leads the higher energy needed for protein Denaturation (biochemistry), denaturation. In general disulfide bonds are necessary in proteins functioning outside cellular space, and they do not change proteins' conformation (geometry), but serve as its stabilizers. Within cytoplasm cysteine residues of proteins are saved in reduced state (i.e. in -SH form) by thioredoxins.
This property manifests in following examples. Lysozyme is stable enough to be applied as a drug. Feathers and hair have relative strength, and consisting in them
Proteins, to execute their Protein#Cellular functions, biological function, need to have specific space geometry. Formation of this geometry is performed in a process called protein folding, and is provided by intra- and inter-molecular bonds. The process has several stages. While at premier stages a polypeptide chain folds due to hydrogen bonds, at later stages folding is provided (apart from hydrogen bonds) by covalent bonds between two sulfur atoms of two cysteine residues (so called disulfide bridges) at different places of a chain (tertriary protein structure) as well as between two cysteine residues in two separated protein subunits (quaternary protein structure). Both structures easily may be seen in insulin. As the bond energy of a covalent disulfide bridge is higher than the energy of a Coordinate covalent bond, coordinate bond or hydrophylic either hydrophobic interaction, the higher disulfide bridges content leads the higher energy needed for protein Denaturation (biochemistry), denaturation. In general disulfide bonds are necessary in proteins functioning outside cellular space, and they do not change proteins' conformation (geometry), but serve as its stabilizers. Within cytoplasm cysteine residues of proteins are saved in reduced state (i.e. in -SH form) by thioredoxins.
This property manifests in following examples. Lysozyme is stable enough to be applied as a drug. Feathers and hair have relative strength, and consisting in them
 Elemental sulfur is non-toxic when one touches it, however it is not harmless. Inhaling sulfur dust, or its contacting to eyes and/or skin may cause irritation. Ingesting sulfur is not safe too. There are reports of cases where people deliberately consumed sulfur (as a folk remedy) that led to life-threatening metabolic acidosis.
Elemental sulfur is non-toxic when one touches it, however it is not harmless. Inhaling sulfur dust, or its contacting to eyes and/or skin may cause irritation. Ingesting sulfur is not safe too. There are reports of cases where people deliberately consumed sulfur (as a folk remedy) that led to life-threatening metabolic acidosis.
Sulfur
at ''The Periodic Table of Videos'' (University of Nottingham)
Atomic Data for Sulfur
NIST Physical Measurement Laboratory
Sulfur phase diagram
, Introduction to Chemistry for Ages 13–17
*[http://extoxnet.orst.edu/pips/sulfur.htm Sulfur and its use as a pesticide]
The Sulphur InstituteNutrient Stewardship and The Sulphur Institute
{{Authority control Sulfur, Chemical elements Chalcogens Reactive nonmetals Polyatomic nonmetals Agricultural chemicals Anti-acne preparations Dietary minerals Industrial minerals Inorganic polymers Native element minerals Orthorhombic minerals Minerals in space group 70 Pyrotechnic fuels Chemical elements with primitive orthorhombic structure
CNRS (
Sicily
(man) it, Siciliana (woman)
, population_note =
, population_blank1_title =
, population_blank1 =
, demographics_type1 = Ethnicity
, demographics1_footnotes =
, demographi ...
were the dominant source for more than a century. By the late 18th century, about 2,000 tonnes per year of sulfur were imported into Marseille
Marseille ( , , ; also spelled in English as Marseilles; oc, Marselha ) is the prefecture of the French department of Bouches-du-Rhône and capital of the Provence-Alpes-Côte d'Azur region. Situated in the camargue region of southern Fra ...
, France, for the production of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
for use in the Leblanc process
The Leblanc process (pronounced leh-blaank) was an early industrial process for making ''soda ash'' (sodium carbonate) used throughout the 19th century, named after its inventor, Nicolas Leblanc. It involved two stages: making sodium sulfate fr ...
. In industrializing
Industrialisation ( alternatively spelled industrialization) is the period of social and economic change that transforms a human group from an agrarian society into an industrial society. This involves an extensive re-organisation of an econ ...
Britain, with the repeal of tariff
A tariff is a tax imposed by the government of a country or by a supranational union on imports or exports of goods. Besides being a source of revenue for the government, import duties can also be a form of regulation of foreign trade and p ...
s on salt in 1824, demand for sulfur from Sicily surged upward. The increasing British control and exploitation of the mining, refining, and transportation of the sulfur, coupled with the failure of this lucrative export to transform Sicily's backward and impoverished economy, led to the Sulfur Crisis of 1840, when King Ferdinand II
Ferdinand II ( an, Ferrando; ca, Ferran; eu, Errando; it, Ferdinando; la, Ferdinandus; es, Fernando; 10 March 1452 – 23 January 1516), also called Ferdinand the Catholic (Spanish: ''el Católico''), was King of Aragon and Sardinia from ...
gave a monopoly of the sulfur industry to a French firm, violating an earlier 1816 trade agreement with Britain. A peaceful solution was eventually negotiated by France.
In 1867, elemental sulfur was discovered in underground deposits in Louisiana
Louisiana , group=pronunciation (French: ''La Louisiane'') is a state in the Deep South and South Central regions of the United States. It is the 20th-smallest by area and the 25th most populous of the 50 U.S. states. Louisiana is bord ...
and Texas
Texas (, ; Spanish: ''Texas'', ''Tejas'') is a state in the South Central region of the United States. At 268,596 square miles (695,662 km2), and with more than 29.1 million residents in 2020, it is the second-largest U.S. state by ...
. The highly successful Frasch process was developed to extract this resource.
In the late 18th century, furniture
Furniture refers to movable objects intended to support various human activities such as seating (e.g., stools, chairs, and sofas), eating ( tables), storing items, eating and/or working with an item, and sleeping (e.g., beds and hammocks) ...
makers used molten sulfur to produce decorative inlays. Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points. Pure powdered sulfur was used as a medicinal tonic and laxative.
With the advent of the contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arsenic ...
, the majority of sulfur today is used to make sulfuric acid for a wide range of uses, particularly fertilizer.
In recent times, the main source of sulfur has become petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
and natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
. This is due to the requirement to remove sulfur from fuels in order to prevent acid rain
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but ac ...
, and has resulted in a surplus of sulfur.
Spelling and etymology
''Sulfur'' is derived from the Latin word ', which was Hellenization, Hellenized to ' in the erroneous belief that the Latin word came from Greek. This spelling was later reinterpreted as representing an /f/ sound and resulted in the spelling ', which appears in Latin toward the end of the Classical antiquity, Classical period. The true Ancient Greek word for sulfur, , ''theîon'' (from earlier , ''théeion''), is the source of the international chemical prefix ''thio-''. The Modern Standard Greek word for sulfur is θείο, ''theío''. In 12th-century Anglo-Norman language, Anglo-French, it was '. In the 14th century, the erroneously Hellenized Latin ' was restored in Middle English '. By the 15th century, both full Latin spelling variants ''sulfur'' and ''sulphur'' became common in English. The parallel ''f~ph'' spellings continued in Britain until the 19th century, when the word was standardized as ''sulphur''. On the other hand, ''sulfur'' was the form chosen in the United States, whereas Canada uses both. The International Union of Pure and Applied Chemistry, IUPAC adopted the spelling ''sulfur'' in 1990 or 1971, depending on the source cited, as did the Nomenclature Committee of the Royal Society of Chemistry in 1992, restoring the spelling ''sulfur'' to Britain. Oxford Dictionaries note that "in chemistry and other technical uses ... the ''-f-'' spelling is now the standard form for this and related words in British as well as US contexts, and is increasingly used in general contexts as well."Production
 Sulfur may be found by itself and historically was usually obtained in this form;
Sulfur may be found by itself and historically was usually obtained in this form; pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Iron, FeSulfur, S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic Luster (mineralogy), lust ...
has also been a source of sulfur. In volcanic regions in Sicily
(man) it, Siciliana (woman)
, population_note =
, population_blank1_title =
, population_blank1 =
, demographics_type1 = Ethnicity
, demographics1_footnotes =
, demographi ...
, in ancient times, it was found on the surface of the Earth, and the "Sicilian method, Sicilian process" was used: sulfur deposits were piled and stacked in brick kilns built on sloping hillsides, with airspaces between them. Then, some sulfur was pulverized, spread over the stacked ore and ignited, causing the free sulfur to melt down the hills. Eventually the surface-borne deposits played out, and miners excavated veins that ultimately dotted the Sicilian landscape with labyrinthine mines. Mining was unmechanized and labor-intensive, with pickmen freeing the ore from the rock, and mine-boys or ''carusu, carusi'' carrying baskets of ore to the surface, often through a mile or more of tunnels. Once the ore was at the surface, it was reduced and extracted in smelting ovens. The conditions in Sicilian sulfur mines were horrific, prompting Booker T. Washington to write "I am not prepared just now to say to what extent I believe in a physical hell in the next world, but a sulphur mine in Sicily is about the nearest thing to hell that I expect to see in this life."
 Elemental sulfur was extracted from
Elemental sulfur was extracted from salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered usi ...
s (in which it sometimes occurs in nearly pure form) until the late 20th century. Sulfur is now produced as a side product of other industrial processes such as in oil refining, in which sulfur is undesired. As a mineral, native sulfur under salt domes is thought to be a fossil mineral resource, produced by the action of anaerobic bacteria on sulfate deposits. It was removed from such salt-dome mines mainly by the Frasch process. In this method, superheated water was pumped into a native sulfur deposit to melt the sulfur, and then compressed air returned the 99.5% pure melted product to the surface. Throughout the 20th century this procedure produced elemental sulfur that required no further purification. Due to a limited number of such sulfur deposits and the high cost of working them, this process for mining sulfur has not been employed in a major way anywhere in the world since 2002.
Today, sulfur is produced from petroleum, natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
, and related fossil resources, from which it is obtained mainly as hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
. Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C–S bonds:
:R-S-R + 2 H2 → 2 RH + H2S
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process. This process entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
:3 O2 + 2 H2S → 2 SO2 + 2 H2O
:SO2 + 2 H2S → 3 S + 2 H2O
Applications
Sulfuric acid
Elemental sulfur is used mainly as a precursor to other chemicals. Approximately 85% (1989) is converted tosulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
(H2SO4):
:2 S + 3 O2 + 2 H2O → 2 H2SO4
 In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
Other important sulfur chemistry
Sulfur reacts directly with methane to givecarbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
, which is used to manufacture cellophane and rayon
Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. It is also called viscose. Many types and grades of viscose ...
. One of the uses of elemental sulfur is in vulcanization of rubber, where polysulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anions, which have the general formu ...
chains crosslink organic polymers. Large quantities of sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are w ...
s are used to bleach (chemical), bleach paper and to preserve dried fruit. Many surfactants and detergents (e.g. sodium lauryl sulfate) are sulfate derivatives. Calcium sulfate, gypsum, (CaSO4·2H2O) is mined on the scale of 100 million tonnes each year for use in Portland cement and fertilizers.
When silver-based photography was widespread, sodium and ammonium sodium thiosulfate, thiosulfate were widely used as "fixing agents". Sulfur is a component of gunpowder ("black powder").
Fertilizer
Amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s synthesized by Organism, living organisms such as methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ...
and cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
contain Organosulfur compounds, organosulfur groups (thioester and thiol respectively). The antioxidant glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
protecting many living organisms against free radicals and oxidative stress also contains organic sulfur. Some crops such as onion and garlic also produce different organosulfur compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulf ...
such as syn-Propanethial-S-oxide, syn-propanethial-S-oxide responsible of lacrymal irritation (onions), or diallyl disulfide and allicin (garlic). Sulfates, commonly found in soils and groundwaters are often a sufficient natural source of sulfur for plants and bacteria. Deposition (aerosol physics), Atmospheric deposition of sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
(SO2) is also a common artificial source (Coal combustion products, coal combustion) of sulfur for the soils. Under normal circumstances, in most agricultural soils, sulfur is not a Limiting factor, limiting nutrient for plants and microorganisms (see the Liebig's law of the minimum#Liebig's barrel). However, in some circumstance, soils can be depleted in sulfate, e.g. if this later is leached by meteoric water (rain) or if the requirements in sulfur for some types of crops are high. This explains that sulfur is increasingly recognized and used as a component of fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
s. The most important form of sulfur for fertilizer is the calcium sulfate, commonly found in nature as the mineral gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywa ...
(CaSO4·2H2O). Elemental sulfur is hydrophobic (not soluble in water) and cannot be used directly by plants. Elemental sulfur (ES) is sometimes mixed with bentonite to amend depleted soils for crops with high requirement in organo-sulfur. Over time, Redox, oxidation Abiotic component, abiotic processes with Earth atmosphere, atmospheric oxygen and Soil microbiology, soil bacteria can Redox, oxidize and convert elemental sulfur to soluble derivatives, which can then be used by microorganisms and plants. Sulfur improves the efficiency of other essential plant nutrients, particularly Nitrate, nitrogen and phosphorus. Biologically produced sulfur particles are naturally Hydrophile, hydrophilic due to a biopolymer coating and are easier to disperse over the land in a spray of diluted slurry, resulting in a faster uptake by plants.
The plants requirement for sulfur equals or exceeds the requirement for phosphorus. It is an plant nutrition, essential nutrient for plant growth, root nodule formation of legumes, and immunity and defense systems. Sulfur deficiency has become widespread in many countries in Europe. Because atmospheric inputs of sulfur continue to decrease, the deficit in the sulfur input/output is likely to increase unless sulfur fertilizers are used. Atmospheric inputs of sulfur decrease because of actions taken to limit acid rain
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but ac ...
s.
Fungicide and pesticide
 Elemental sulfur is one of the oldest fungicides and pesticides. "Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organic agriculture, organically farmed apple production against the main disease apple scab under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or Aerial application, from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscibility, miscible. It has similar applications and is used as a
Elemental sulfur is one of the oldest fungicides and pesticides. "Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organic agriculture, organically farmed apple production against the main disease apple scab under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or Aerial application, from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscibility, miscible. It has similar applications and is used as a fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality ...
against mildew and other mold-related problems with plants and soil.
Elemental sulfur powder is used as an "organic farming, organic" (i.e., "green") insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed t ...
(actually an acaricide) against ticks and mites. A common method of application is dusting the clothing or limbs with sulfur powder.
A diluted solution of lime sulfur (made by combining calcium hydroxide with elemental sulfur in water) is used as a dip for pets to destroy ringworm, ringworm (fungus), mange, and other cutaneous conditions, dermatoses and parasitism, parasites.
Sulfur candles of almost pure sulfur were burned to fumigant, fumigate structures and wine barrels, but are now considered too toxic for residences.
Pharmaceuticals
Sulfur (specificallyoctasulfur
Octasulfur is an inorganic substance with the chemical formula . It is an odourless and tasteless yellow solid, and is a major industrial chemical. It is the most common allotrope of sulfur and occurs widely in nature.Steudel, R., "Homocyclic Sul ...
, S8) is used in pharmaceutical skin preparations for the treatment of acne
Acne, also known as ''acne vulgaris'', is a long-term skin condition that occurs when dead skin cells and oil from the skin clog hair follicles. Typical features of the condition include blackheads or whiteheads, pimples, oily skin, and ...
and other conditions. It acts as a keratolytic agent and also kills bacteria, fungi, scabies
Scabies (; also sometimes known as the seven-year itch) is a contagious skin infestation by the mite ''Sarcoptes scabiei''. The most common symptoms are severe itchiness and a pimple-like rash. Occasionally, tiny burrows may appear on the ski ...
mites, and other parasites. Precipitated sulfur and colloidal sulfur are used, in form of lotions, creams, powders, soaps, and bath additives, for the treatment of acne vulgaris, acne rosacea, and seborrhoeic dermatitis.
Many drugs contain sulfur. Early examples include antibacterial sulfonamide (medicine), sulfonamides, known as ''sulfa drugs''. A more recent example is mucolytic acetylcysteine. Sulfur is a part of many bacterial defense molecules. Most β-lactam antibiotics, including the penicillins, cephalosporins and monobactams contain sulfur.
Batteries
Due to their high energy density and the availability of sulfur, there is ongoing research in creating rechargeable Lithium–sulfur battery, lithium-sulfur batteries. Until now, carbonate electrolytes have caused failures in such batteries after a single cycle. In February 2022, researchers at Drexel University have not only created a prototypical battery that lasted 4000 recharge cycles, but also found the first monoclinic gamma sulfur that remained stable below 95 degrees Celsius.Biological role
Sulfur is an essential component of all living cell (biology), cells. It is the eighth most abundant element in the human body by weight, about equal in abundance to potassium, and slightly greater than sodium and chlorine. A human body contains about 140 grams of sulfur. The main dietary source of sulfur for humans is sulfur-containing amino-acids, which can be found in plant and animal proteins.Transferring sulfur between inorganic and biomolecules
In the 1880s, while studying Beggiatoa (a bacterium living in a sulfur rich environment), Sergei Winogradsky found that it oxidizedhydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
(H2S) as an energy source, forming intracellular sulfur droplets. Winogradsky referred to this form of metabolism as inorgoxidation (oxidation of inorganic compounds). Another contributor, who continued to study it was Selman Waksman. Primitive bacteria that live around deep ocean hydrothermal vent, volcanic vents oxidize hydrogen sulfide for their nutrition, as discovered by Robert Ballard.
Sulfur oxidizers can use as energy sources reduced sulfur compounds, including hydrogen sulfide, elemental sulfur, sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are w ...
, thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e ...
, and various polythionates (e.g., tetrathionate). They depend on enzymes such as sulfur dioxygenase, sulfur oxygenase and sulfite oxidase to oxidize sulfur to sulfate. Some lithotrophs can even use the energy contained in sulfur compounds to produce sugars, a process known as chemosynthesis. Some bacteria and archaea use hydrogen sulfide in place of water as the electron donor in chemosynthesis, a process similar to photosynthesis that produces sugars and uses oxygen as the electron acceptor. Sulfur-based chemosynthesis may be simplifiedly compared with photosynthesis:
: H2S +CO2 → sugars + S
: H2O + CO2 → sugars + O2
There are bacteria combining these two ways of nutrition: green sulfur bacteria and purple sulfur bacteria. Also sulfur-oxidizing bacteria can go into symbiosis with larger organisms, enabling the later to use hydrogen sulfide as food to be oxidized. Example: the giant tube worm.
There are sulfate-reducing bacteria, that, by contrast, "breathe sulfate" instead of oxygen. They use organic compounds or molecular hydrogen as the energy source. They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They can grow on other partially oxidized sulfur compounds (e.g. thiosulfates, thionates, polysulfides, sulfites).
There are studies pointing that many deposits of native sulfur in places that were the bottom of Tethys Ocean, the ancient oceans have biological origin. These studies indicate that this native sulfur have been obtained through biological activity, but what is responsible for that (sulfur-oxidizing bacteria or sulfate-reducing bacteria) is still unknown for sure.
Sulfur is absorbed by plants roots from soil as sulfate and transported as a phosphate ester. Sulfate is reduced to sulfide via sulfite before it is incorporated into cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
and other organosulfur compounds.
: SO42− → SO32− → H2S → cysteine (thiol) → methionine (thioether)
While the plants' role in transferring sulfur to animals by food chains is more or less understood, the role of sulfur bacteria is just getting investigated.
Protein and organic metabolites
In all forms of life, most of the sulfur is contained in two proteinogenic amino acids (cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
and methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ...
), thus the element is present in all proteins that contain these amino acids, as well as in respective peptides. Some of the sulfur is comprised in certain metabolites — many of which are Cofactor (biochemistry), cofactors, — and sulfated polysaccharides of connective tissue (chondroitin sulfates, heparin).
 Proteins, to execute their Protein#Cellular functions, biological function, need to have specific space geometry. Formation of this geometry is performed in a process called protein folding, and is provided by intra- and inter-molecular bonds. The process has several stages. While at premier stages a polypeptide chain folds due to hydrogen bonds, at later stages folding is provided (apart from hydrogen bonds) by covalent bonds between two sulfur atoms of two cysteine residues (so called disulfide bridges) at different places of a chain (tertriary protein structure) as well as between two cysteine residues in two separated protein subunits (quaternary protein structure). Both structures easily may be seen in insulin. As the bond energy of a covalent disulfide bridge is higher than the energy of a Coordinate covalent bond, coordinate bond or hydrophylic either hydrophobic interaction, the higher disulfide bridges content leads the higher energy needed for protein Denaturation (biochemistry), denaturation. In general disulfide bonds are necessary in proteins functioning outside cellular space, and they do not change proteins' conformation (geometry), but serve as its stabilizers. Within cytoplasm cysteine residues of proteins are saved in reduced state (i.e. in -SH form) by thioredoxins.
This property manifests in following examples. Lysozyme is stable enough to be applied as a drug. Feathers and hair have relative strength, and consisting in them
Proteins, to execute their Protein#Cellular functions, biological function, need to have specific space geometry. Formation of this geometry is performed in a process called protein folding, and is provided by intra- and inter-molecular bonds. The process has several stages. While at premier stages a polypeptide chain folds due to hydrogen bonds, at later stages folding is provided (apart from hydrogen bonds) by covalent bonds between two sulfur atoms of two cysteine residues (so called disulfide bridges) at different places of a chain (tertriary protein structure) as well as between two cysteine residues in two separated protein subunits (quaternary protein structure). Both structures easily may be seen in insulin. As the bond energy of a covalent disulfide bridge is higher than the energy of a Coordinate covalent bond, coordinate bond or hydrophylic either hydrophobic interaction, the higher disulfide bridges content leads the higher energy needed for protein Denaturation (biochemistry), denaturation. In general disulfide bonds are necessary in proteins functioning outside cellular space, and they do not change proteins' conformation (geometry), but serve as its stabilizers. Within cytoplasm cysteine residues of proteins are saved in reduced state (i.e. in -SH form) by thioredoxins.
This property manifests in following examples. Lysozyme is stable enough to be applied as a drug. Feathers and hair have relative strength, and consisting in them keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up Scale (anatomy), scales, hair, Nail ...
is considered indigestible by most organisms. However, there are fungi and bacteria containing keratinase, and are able to destruct keratin.
Many important cellular enzymes use prosthetic groups ending with -SH moieties to handle reactions involving acyl-containing biochemicals: two common examples from basic metabolism are coenzyme A and alpha-lipoic acid. Cysteine-related metabolites homocysteine and taurine
Taurine (), or 2-aminoethanesulfonic acid, is an organic compound that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight. I ...
are other sulfur-containing amino acids that are similar in structure, but not coded by DNA, and are not part of the primary structure of proteins, take part in various locations of mammalian physiology. Two of the 13 classical vitamins, biotin
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', bo ...
, and thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, that cannot be made in the body. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thi ...
, contain sulfur, and serve as cofactors to several enzymes.
In intracellular chemistry, sulfur operates as a carrier of reducing hydrogen and its electrons for cellular repair of oxidation. Reduced glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
, a sulfur-containing tripeptide, is a reducing agent through its sulfhydryl (-SH) moiety derived from cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
.
Methanogenesis, the route to most of the world's methane, is a multistep biochemical transformation of carbon dioxide. This conversion requires several organosulfur cofactors. These include coenzyme M, CH3SCH2CH2SO3−, the immediate precursor to methane.
Metalloproteins and inorganic cofactors
Metalloproteins — in which the active site is a transition metal ion (or metal-sulfide cluster) often coordinated by sulfur atoms of cysteine residues — are essential components of enzymes involved in electron transfer processes. Examples include plastocyanin (Cu2+) and nitrous-oxide reductase, nitrous oxide reductase (Cu–S). The function of these enzymes is dependent on the fact that the transition metal ion can undergo redox reactions. Other examples include many zinc proteins, as well as iron–sulfur clusters. Most pervasive are the ferrodoxins, which serve as electron shuttles in cells. In bacteria, the important nitrogenase enzymes contains an Fe–Mo–S cluster and is a catalyst that performs the important function of nitrogen fixation, converting atmospheric nitrogen to ammonia that can be used by microorganisms and plants to make proteins, DNA, RNA, alkaloids, and the other organic nitrogen compounds necessary for life. :
Deficiency
In humans methionine is an essential amino acid, cysteine is conditionally essential and may be synthesized from non-essential serine (sulfur donator would be methionine in this case). Dietary deficiency rarely happens in common conditions. Artificial methionine deficiency is attempted to apply in cancer treatment, but the method is still potentially dangerous. There is a rare fatal genetic disease connected with sulfite oxidase impairment an enzyme metabolizing sulfur-containing amino acids.Precautions
Toxicity of sulfur compounds
Most of the soluble sulfate salts, such as Epsom salts, are non-toxic. Soluble sulfate salts are poorly absorbed and laxative. When injected parenterally, they are freely filtered by the kidneys and eliminated with very little toxicity in multi-gram amounts. Aluminium sulfate is used in the purification of drinking water, wastewater treatment plants and papermaking. When sulfur burns in air, it producessulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
. In water, this gas produces sulfurous acid and sulfites; sulfites are antioxidants that inhibit growth of aerobic bacteria and a useful food additive in small amounts. At high concentrations these acids harm the human lungs, lungs, human eyes, eyes, or other biological tissue, tissues. In organisms without lungs such as insects or plants, sulfite in high concentration prevents respiration (physiology), respiration.
Sulfur trioxide (made by catalysis from sulfur dioxide) and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
are similarly highly acidic and corrosive in the presence of water. Sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic tissue.
The burning of coal and/or petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
by industry and power plants generates sulfur dioxide (SO2) that reacts with atmospheric water and oxygen to produce sulfuric acid (H2SO4) and sulfurous acid (H2SO3). These acids are components of acid rain
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but ac ...
, lowering the pH of soil and freshwater bodies, sometimes resulting in substantial damage to the environment (biophysical), environment and chemical weathering of statues and structures. Fuel standards increasingly require that fuel producers extract sulfur from fossil fuels to prevent acid rain formation. This extracted and refined sulfur represents a large portion of sulfur production. In coal-fired power plants, flue gases are sometimes purified. More modern power plants that use synthesis gas extract the sulfur before they burn the gas.
Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
is about one-half as toxic as hydrogen cyanide, and intoxicates by the same mechanism (inhibition of the respiratory enzyme cytochrome oxidase), though hydrogen sulfide is less likely to cause sudden poisonings from small inhaled amounts (near its permissible exposure limit — PEL — of 20 ppm) because of its disagreeable odor. However, its presence in ambient air at concentration over 100-150 ppm quickly deadens the sense of smell, and a victim may breathe increasing quantities without noticing until severe symptoms cause death. Dissolved sulfide and hydrosulfide salts are toxic by the same mechanism.
See also
*Blue lava *Stratospheric sulfur aerosols *Sulfur assimilation *Ultra-low sulfur dieselReferences
Further reading
External links
Sulfur
at ''The Periodic Table of Videos'' (University of Nottingham)
Atomic Data for Sulfur
NIST Physical Measurement Laboratory
Sulfur phase diagram
, Introduction to Chemistry for Ages 13–17
*[http://extoxnet.orst.edu/pips/sulfur.htm Sulfur and its use as a pesticide]
The Sulphur Institute
{{Authority control Sulfur, Chemical elements Chalcogens Reactive nonmetals Polyatomic nonmetals Agricultural chemicals Anti-acne preparations Dietary minerals Industrial minerals Inorganic polymers Native element minerals Orthorhombic minerals Minerals in space group 70 Pyrotechnic fuels Chemical elements with primitive orthorhombic structure