Sulfate-reducing on:
[Wikipedia]
[Google]
[Amazon]
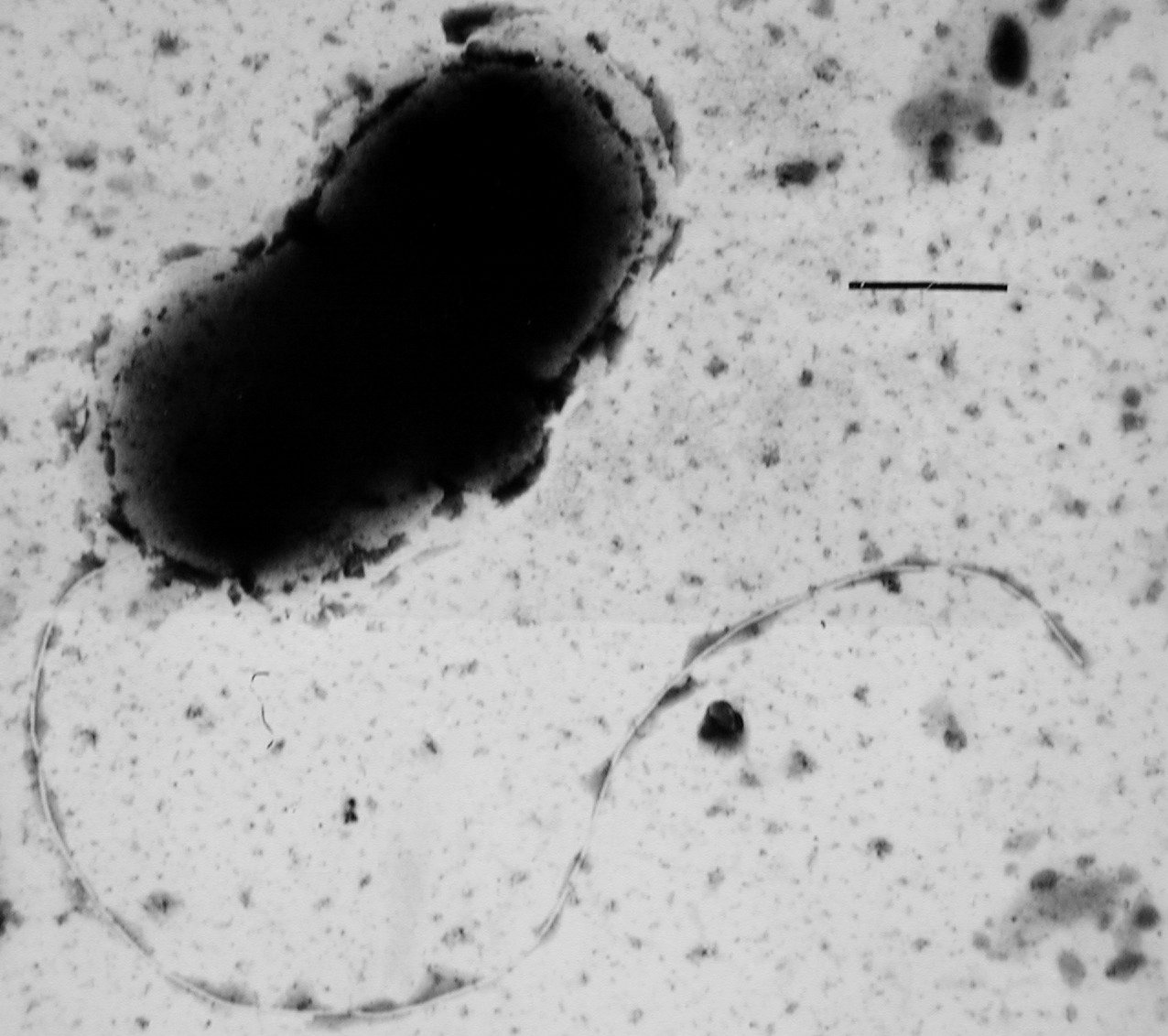 Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing
 The toxic
The toxic
 The enzyme dissimilatory (bi)sulfite reductase, ''dsrAB'' (EC 1.8.99.5), that catalyzes the last step of dissimilatory sulfate reduction, is the functional gene most used as a molecular marker to detect the presence of sulfate-reducing microorganisms.
The enzyme dissimilatory (bi)sulfite reductase, ''dsrAB'' (EC 1.8.99.5), that catalyzes the last step of dissimilatory sulfate reduction, is the functional gene most used as a molecular marker to detect the presence of sulfate-reducing microorganisms.
Oldest Water on Earth Found Deep Within the Canadian Shield
December 14, 2016, Maggie Romuld
'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory
Garnet S. Lollar, Oliver Warr, Jon Telling, Magdalena R. Osburn & Barbara Sherwood Lollar, Received 15 Jan 2019, Accepted 01 Jul 2019, Published online: 18 Jul 2019.
Deep fracture fluids isolated in the crust since the Precambrian era
G. Holland, B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G. F. Slater & C. J. Ballentine, Nature volume 497, pages 357–360 (16 May 2013)
Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks
by L. Li, B. A. Wing, T. H. Bui, J. M. McDermott, G. F. Slater, S. Wei, G. Lacrampe-Couloume & B. Sherwood Lollar October 27, 2016. Nature Communications volume 7, Article number: 13252 (2016.)
Earth's mysterious 'deep biosphere' may harbor millions of undiscovered species
By Brandon Specktor, Live Science, December 11, 2018, published online at nbcnews.com. {{DEFAULTSORT:Sulfate-Reducing Bacteria Bacteria Martinus Beijerinck Extremophiles Environmental microbiology Ecology Geomicrobiology Microbial growth and nutrition
archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
(SRA), both of which can perform anaerobic respiration utilizing sulfate () as terminal electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
, reducing it to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
(H2S). Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than molecular oxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
(O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
.
Most sulfate-reducing microorganisms can also reduce some other oxidized inorganic sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
compounds, such as sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are wide ...
(), dithionite
The dithionite is the oxyanion with the formula 2O4sup>2−. It is commonly encountered as the salt sodium dithionite. For historical reasons, it is sometimes called hydrosulfite, but it contains no hydrogen and is not a sulfite. The diani ...
(), thiosulfate (), trithionate (), tetrathionate (), elemental sulfur (S8), and polysulfides (). Depending on the context, "sulfate-reducing microorganisms" can be used in a broader sense (including all species that can reduce any of these sulfur compounds) or in a narrower sense (including only species that reduce sulfate, and excluding strict thiosulfate and sulfur reducers, for example).
Sulfate-reducing microorganisms can be traced back to 3.5 billion years ago and are considered to be among the oldest forms of microbes, having contributed to the sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element ( CHNOPS), being a const ...
soon after life emerged on Earth.
Many organisms reduce small amounts of sulfates in order to synthesize sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
-containing cell components; this is known as ''assimilatory sulfate reduction''. By contrast, the sulfate-reducing microorganisms considered here reduce sulfate in large amounts to obtain energy and expel the resulting sulfide as waste; this is known as dissimilatory sulfate reduction. They use sulfate as the terminal electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
of their electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
. Most of them are anaerobes
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenate ...
; however, there are examples of sulfate-reducing microorganisms that are tolerant of oxygen, and some of them can even perform aerobic respiration.
No growth is observed when oxygen is used as the electron acceptor.
"
In addition, there are sulfate-reducing microorganisms that can also reduce other electron acceptors, such as fumarate
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The salts and esters are known as f ...
, nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
(), nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
(), ferric iron (Fe3+), and dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds a ...
(DMSO).
In terms of electron donor, this group contains both organotrophs and lithotrophs. The organotrophs oxidize organic compounds, such as carbohydrates, organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are rel ...
s (such as formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ''Ull ...
, lactate
Lactate may refer to:
* Lactation, the secretion of milk from the mammary glands
* Lactate, the conjugate base of lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with ...
, acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
, propionate, and butyrate
The conjugate acids are in :Carboxylic acids.
{{Commons category, Carboxylate ions, Carboxylate anions
Carbon compounds
Oxyanions ...
), alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s (methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
and ethanol), aliphatic hydrocarbons
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, o ...
(including methane), and aromatic hydrocarbons ( benzene, toluene, ethylbenzene, and xylene). The lithotrophs oxidize molecular hydrogen (H2), for which they compete with methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are com ...
s and acetogen
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic respi ...
s in anaerobic conditions. Some sulfate-reducing microorganisms can directly use metallic iron (Fe0, also known as zerovalent iron, or ZVI) as electron donor, oxidizing it to ferrous iron (Fe2+).
Ecological importance and markers
Sulfate occurs widely in seawater, sediment, and water rich in decaying organic material. Sulfate is also found in more extreme environments such as hydrothermal vents, acid mine drainage sites, oil fields, and the deep subsurface, including the world's oldest isolated ground water. Sulfate-reducing microorganisms are common in anaerobic environments where they aid in the degradation of organic materials. In these anaerobic environments, fermenting bacteria extract energy from large organic molecules; the resulting smaller compounds such asorganic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are rel ...
s and alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s are further oxidized by acetogen
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic respi ...
s and methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are com ...
s and the competing sulfate-reducing microorganisms.
 The toxic
The toxic hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
is a waste product of sulfate-reducing microorganisms; its rotten egg odor is often a marker for the presence of sulfate-reducing microorganisms in nature.
Sulfate-reducing microorganisms are responsible for the sulfurous odors of salt marshes and mud flats. Much of the hydrogen sulfide will react with metal ions in the water to produce metal sulfides. These metal sulfides, such as ferrous sulfide
Iron(II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family chemical compounds and minerals with the approximate formula . Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.
Preparation ...
(FeS), are insoluble and often black or brown, leading to the dark color of sludge.
During the Permian–Triassic extinction event (250 million years ago) a severe anoxic event seems to have occurred where these forms of bacteria became the dominant force in oceanic ecosystems, producing copious amounts of hydrogen sulfide.
Sulfate-reducing bacteria also generate neurotoxic methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a ...
as a byproduct of their metabolism, through methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
of inorganic mercury present in their surroundings. They are known to be the dominant source of this bioaccumulative form of mercury in aquatic systems.
Uses
Some sulfate-reducing microorganisms can reduce hydrocarbons, and they have been used to clean up contaminated soils. Their use has also been proposed for other kinds of contaminations. Sulfate-reducing microorganisms are considered a possible way to deal with acid mine waters that are produced by other microorganisms.Problems caused by sulfate-reducing microorganisms
In engineering, sulfate-reducing microorganisms can create problems when metal structures are exposed to sulfate-containing water: Interaction of water and metal creates a layer of molecular hydrogen on the metal surface; sulfate-reducing microorganisms then oxidize the hydrogen while creating hydrogen sulfide, which contributes to corrosion. Hydrogen sulfide from sulfate-reducing microorganisms also plays a role in the biogenic sulfide corrosion of concrete. It also occurs in sour crude oil. Some sulfate-reducing microorganisms play a role in the anaerobic oxidation of methane: :CH4 + → + HS− + H2O An important fraction of the methane formed bymethanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are com ...
s below the seabed is oxidized by sulfate-reducing microorganisms in the transition zone separating the methanogenesis from the sulfate reduction activity in the sediments. This process is also considered a major sink for sulfate in marine sediments.
In hydraulic fracturing
Fracking (also known as hydraulic fracturing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of bedrock formations by a pressurized liquid. The process involves the high-pressure injection of "frack ...
, fluids are used to frack shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4) and tiny fragments (silt-sized particles) of other minerals, especial ...
formation
Formation may refer to:
Linguistics
* Back-formation, the process of creating a new lexeme by removing or affixes
* Word formation, the creation of a new word by adding affixes
Mathematics and science
* Cave formation or speleothem, a secondar ...
s to recover methane ( shale gas) and hydrocarbons. Biocide
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slig ...
compounds are often added to water to inhibit the microbial activity of sulfate-reducing microorganisms, in order to but not limited to, avoid anaerobic methane oxidation and the generation of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
, ultimately resulting in minimizing potential production loss.
Biochemistry
Before sulfate can be used as an electron acceptor, it must be activated. This is done by the enzymeATP-sulfurylase
In enzymology, a sulfate adenylyltransferase () is an enzyme that catalyzes the chemical reaction
:ATP + sulfate \rightleftharpoonspyrophosphate + adenylyl sulfate
Thus, the two substrates of this enzyme are ATP and sulfate, whereas its two p ...
, which uses ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
and sulfate to create adenosine 5′-phosphosulfate (APS). APS is subsequently reduced to sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are wide ...
and AMP. Sulfite is then further reduced to sulfide, while AMP is turned into ADP
Adp or ADP may refer to:
Aviation
* Aéroports de Paris, airport authority for the Parisian region in France
* Aeropuertos del Perú, airport operator for airports in northern Peru
* SLAF Anuradhapura, an airport in Sri Lanka
* Ampara Air ...
using another molecule of ATP. The overall process, thus, involves an investment of two molecules of the energy carrier ATP, which must to be regained from the reduction.
Phylogeny
The sulfate-reducing microorganisms have been treated as a phenotypic group, together with the other sulfur-reducing bacteria, for identification purposes. They are found in several different phylogenetic lines. As of 2009, 60 genera containing 220 species of sulfate-reducing bacteria are known. Among the Thermodesulfobacteriota the orders of sulfate-reducing bacteria includeDesulfobacterales
Desulfobacterales are an order of sulfate-reducing bacteria within the phylum Thermodesulfobacteria. The order contains three families; ''Desulfobacteraceae, Desulfobulbaceae'', and ''Nitrospinaceae''. The bacterium in this order are strict anae ...
, Desulfovibrionales, and Syntrophobacterales
The Syntrophobacterales are an order of Thermodesulfobacteriota. All genera are strictly anaerobic.Garrity, George M.; Brenner, Don J.; Krieg, Noel R.; Staley, James T. (eds.) (2005). Bergey's Manual of Systematic Bacteriology, Volume Two: The P ...
. This accounts for the largest group of sulfate-reducing bacteria, about 23 genera.
The second largest group of sulfate-reducing bacteria is found among the Bacillota, including the genera ''Desulfotomaculum
''Desulfotomaculum'' is a genus of Gram-positive, obligately anaerobic soil bacteria. A type of sulfate-reducing bacteria, ''Desulfotomaculum'' can cause food spoilage in poorly processed canned foods. Their presence can be identified by the re ...
'', ''Desulfosporomusa
''Desulfosporomusa'' is a genus of sulfate-reducing bacteria. So far there is only one species of this genus known ('' Desulfosporomusa polytropa'').
References
External links
''Desulfosporomusa''at NCBI
The National Center for Biotechn ...
'', and ''Desulfosporosinus
''Desulfosporosinus'' is a genus of strictly anaerobic, sulfate-reducing bacteria, often found in soil.
The type species ''D. orientis'' was isolated in 1959 with the proposed name ''Desulfovibrio orientis'', and was later assigned to the genus ...
''.
In the Nitrospirota phylum we find sulfate-reducing '' Thermodesulfovibrio'' species.
Two more groups that include thermophilic sulfate-reducing bacteria are given their own phyla, the Thermodesulfobacteriota and ''Thermodesulfobium
''Thermodesulfobium '' is a Gram-negative, strictly Anaerobic organism, anaerobic, moderately thermophilic, non-spore-forming and non-motile genus of bacteria from the family of Thermodesulfobiaceae.
See also
* List of bacteria genera
* List of ...
''.
There are also three known genera of sulfate-reducing archaea: ''Archaeoglobus
''Archaeoglobus'' is a genus of the phylum Euryarchaeota. ''Archaeoglobus'' can be found in high-temperature oil fields where they may contribute to oil field souring.
Metabolism
''Archaeoglobus'' grow anaerobically at extremely high temperat ...
'', ''Thermocladium
In taxonomy, ''Thermocladium'' is a genus of the Thermoproteaceae.See the NCBI
The National Center for Biotechnology Information (NCBI) is part of the United States National Library of Medicine (NLM), a branch of the National Institutes of H ...
'' and ''Caldivirga
In taxonomy, ''Caldivirga'' is a genus of the Thermoproteaceae.See the NCBI
The National Center for Biotechnology Information (NCBI) is part of the United States National Library of Medicine (NLM), a branch of the National Institutes of Hea ...
''. They are found in hydrothermal vents, oil deposits, and hot springs.
In July 2019, a scientific study of Kidd Mine
Kidd Mine or Kidd Creek Mine is an underground base metal mine north of Timmins, Ontario, Canada. It is owned and operated by Swiss multinational Glencore Inc. The mine was discovered in 1963 by Texas Gulf Sulfur Company. In 1981 it was sold to ...
in Canada discovered sulfate-reducing microorganisms living below the surface. The sulfate reducers discovered in Kidd Mine are lithotrophs, obtaining their energy by oxidizing minerals such as pyrite rather than organic compounds. Kidd Mine is also the site of the oldest known water on Earth.December 14, 2016, Maggie Romuld
See also
* Anaerobic respiration * Deep biosphere * Extremophile * Microbial metabolism * Microorganism *Quinone-interacting membrane-bound oxidoreductase Quinone-interacting membrane-bound oxidoreductase is a membrane-bound protein complex present in the electron transport chain of Sulfate-reducing bacteria, sulfate reducers (e.g. ''Desulfovibrio'' species) and some sulfur oxidizers.
It was first de ...
* Sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element ( CHNOPS), being a const ...
References
External links
'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory
Garnet S. Lollar, Oliver Warr, Jon Telling, Magdalena R. Osburn & Barbara Sherwood Lollar, Received 15 Jan 2019, Accepted 01 Jul 2019, Published online: 18 Jul 2019.
Deep fracture fluids isolated in the crust since the Precambrian era
G. Holland, B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G. F. Slater & C. J. Ballentine, Nature volume 497, pages 357–360 (16 May 2013)
Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks
by L. Li, B. A. Wing, T. H. Bui, J. M. McDermott, G. F. Slater, S. Wei, G. Lacrampe-Couloume & B. Sherwood Lollar October 27, 2016. Nature Communications volume 7, Article number: 13252 (2016.)
Earth's mysterious 'deep biosphere' may harbor millions of undiscovered species
By Brandon Specktor, Live Science, December 11, 2018, published online at nbcnews.com. {{DEFAULTSORT:Sulfate-Reducing Bacteria Bacteria Martinus Beijerinck Extremophiles Environmental microbiology Ecology Geomicrobiology Microbial growth and nutrition