stopped-flow on:
[Wikipedia]
[Google]
[Amazon]
Stopped-flow is an experimental technique for studying chemical reactions with a half time of the order of 1 ms, introduced by

 Stopped-flow spectrometry allows
Stopped-flow spectrometry allows
 Once the two solutions are forced out of their syringes they enter a mixing system that has baffles to ensure complete mixing, with turbulent flow rather than laminar flow, which would allow the two solutions to flow side by side with incomplete mixing.
Once the two solutions are forced out of their syringes they enter a mixing system that has baffles to ensure complete mixing, with turbulent flow rather than laminar flow, which would allow the two solutions to flow side by side with incomplete mixing.
 The mixed reactants pass an observation cell that allows the reaction to be followed spectrophotometrically, typically by
The mixed reactants pass an observation cell that allows the reaction to be followed spectrophotometrically, typically by
 The stopped-flow method is a development of the continuous-flow method used by
The stopped-flow method is a development of the continuous-flow method used by
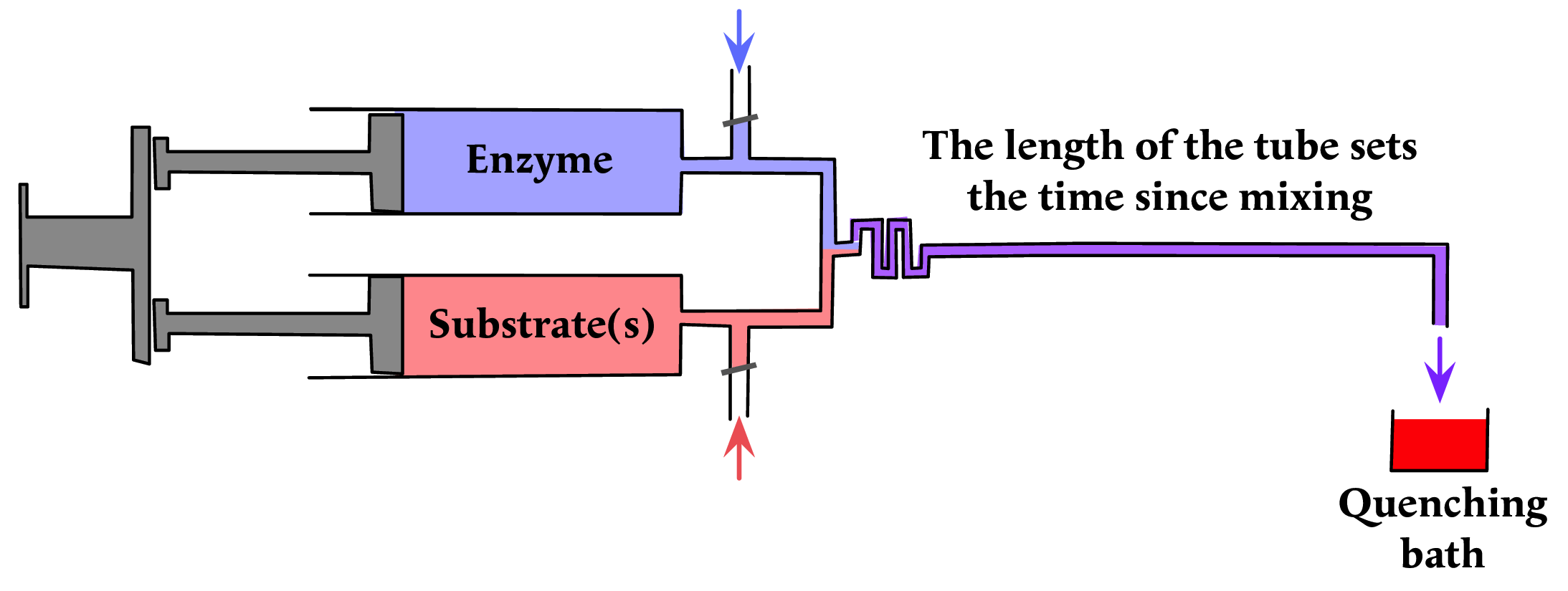 The stopped-flow method depends on the existence of spectroscopic properties that can be used for following the reaction. When that is not the case quenched flow provides an alternative that uses conventional chemical methods for analysis. Instead of a mechanical stopping system the reaction is stopped by ''quenching'', the products being delivered to a recipient that stops the reaction immediately, either by instantaneous freezing or by denaturing the enzyme with a chemical denaturant or exposing the sample to a denaturing light source. As in the continuous-flow method, the time between mixing and quenching can be varied by varying the length of the tube.
The stopped-flow method depends on the existence of spectroscopic properties that can be used for following the reaction. When that is not the case quenched flow provides an alternative that uses conventional chemical methods for analysis. Instead of a mechanical stopping system the reaction is stopped by ''quenching'', the products being delivered to a recipient that stops the reaction immediately, either by instantaneous freezing or by denaturing the enzyme with a chemical denaturant or exposing the sample to a denaturing light source. As in the continuous-flow method, the time between mixing and quenching can be varied by varying the length of the tube.
 The ''pulsed quenched flow'' method introduced by
The ''pulsed quenched flow'' method introduced by
Britton Chance
Britton "Brit" Chance (July 24, 1913 – November 16, 2010) was an American biochemist, biophysicist, scholar, and inventor whose work helped develop spectroscopy as a way to diagnose medical problems. He was "a world leader in transforming t ...
and extended by Quentin Gibson
Quentin Howieson Gibson FRS (9 December 1918 – 16 March 2011) was a Scottish American physiologist, and professor at University of Sheffield, and Cornell University.
Education
Gibson earned a Doctor of Medicine degree in 1944 and a Ph.D. in 19 ...
(Other techniques, such as the temperature-jump method, are available for much faster processes.)
Description of the method
Summary

 Stopped-flow spectrometry allows
Stopped-flow spectrometry allows chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in wh ...
of fast reactions (with half times of the order of milliseconds) to be studied in solution. It was first especially used to enzyme-catalyzed reactions. Then the stopped-flow rapidly found its place in almost all biochemistry, biophysics, and chemistry laboratories with a need to follow chemical reactions in the millisecond time scale.
In its simplest form, a stopped-flow mixes two solutions. Small volumes of solutions are rapidly and continuously driven into a high-efficiency mixer. This mixing process then initiates an extremely fast reaction. The newly mixed solution travels to the observation cell and pushes out the contents of the cell (the solution remaining from the previous experiment or from necessary washing steps). The time required for this solution to pass from the mixing point to the observation point is known as dead time. The minimum injection volume will depend on the volume of the mixing cell. Once enough solution has been injected to completely remove the previous solution, the instrument reaches a stationary state and the flow can be stopped. Depending on the syringe drive technology, the flow stop is achieved by using a stop valve called the hard-stop or by using a stop syringe. The stopped-flow also sends a ‘start signal’ to the detector called the trigger so the reaction can be observed. The timing of the trigger is usually software controlled so the user can trigger at the same time the flow stops or a few milliseconds before the stop to check the stationary state has been reached.
So this is a very economical technique.
Reactant syringes
Two syringes are filled with solutions that do not undergo a chemical reaction until mixed together. These have pistons that are driven by a single drive piston or by independent stepping motors, so that they are coupled together and their contents are forced out simultaneously into a mixing device.Mixing chamber
 Once the two solutions are forced out of their syringes they enter a mixing system that has baffles to ensure complete mixing, with turbulent flow rather than laminar flow, which would allow the two solutions to flow side by side with incomplete mixing.
Once the two solutions are forced out of their syringes they enter a mixing system that has baffles to ensure complete mixing, with turbulent flow rather than laminar flow, which would allow the two solutions to flow side by side with incomplete mixing.
Dead time
The dead time is the time for the solutions to go from the mixing point to the observation point, it is the part of the kinetics which cannot be observed. So the lower the dead time, the more information the user can get. In older instruments this could be of the order of 1 ms, but improvements now allow a dead time of about 0.3 ms.Observation cell
 The mixed reactants pass an observation cell that allows the reaction to be followed spectrophotometrically, typically by
The mixed reactants pass an observation cell that allows the reaction to be followed spectrophotometrically, typically by ultraviolet spectroscopy
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
, fluorescence spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electron ...
, circular dichroism or light scattering
Scattering is a term used in physics to describe a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including ...
, and it is now common to combine several of these.
Observation cuvette with a short light path (0.75 to 1.5mm) are usually preferred for fluorescence measurements to reduce self-absorption effects. Observation cuvette with longer light path (0.5cm to 1cm) are preferred for absorbance measurements. Modern stopped-flow can accommodate different models of cells and it is possible to change the cuvette between two experiments.
For stopped-flow X-ray measurements, a quartz capillary with thin wall is used to minimize quartz absorption. Simultaneous x-ray and absorbance measurements are possible in the same capillary.
Stopping
Once through the observation cell the mixture enters a third syringe that contains a piston that is driven by the flow to activate a switch to stop the flow and activate the observation.Continuous flow
 The stopped-flow method is a development of the continuous-flow method used by
The stopped-flow method is a development of the continuous-flow method used by Hamilton Hartridge
Hamilton Hartridge (7 May 1886 – 13 January 1976) was a British eye physiologist and medical writer.'Obituary: H. Hartridge', ''British Medical Journal'', 20 March 1976, p.716 Known for his ingenious experimentation and instrument construction ...
and Francis Roughton to study the binding of O2 to hemoglobin. In the absence of any stopping system the reaction mixture passed to a long tube past an observation system (consisting in 1923 of a simple colorimeter) to waste. By moving the colorimeter along the tube, and knowing the flow rate, Hartridge and Roughton could measure the process after a known time.
In its time this was a revolutionary advance showing an apparently intractable problem (studying a process taking milliseconds with equipment that required seconds for each measurement) could be solved with simple equipment. However in practice it was limited to reactants available in large quantities: for proteins this effectively limited it to reactions of hemoglobin. For practical purposes this approach is obsolete.
Quenched flow
 The stopped-flow method depends on the existence of spectroscopic properties that can be used for following the reaction. When that is not the case quenched flow provides an alternative that uses conventional chemical methods for analysis. Instead of a mechanical stopping system the reaction is stopped by ''quenching'', the products being delivered to a recipient that stops the reaction immediately, either by instantaneous freezing or by denaturing the enzyme with a chemical denaturant or exposing the sample to a denaturing light source. As in the continuous-flow method, the time between mixing and quenching can be varied by varying the length of the tube.
The stopped-flow method depends on the existence of spectroscopic properties that can be used for following the reaction. When that is not the case quenched flow provides an alternative that uses conventional chemical methods for analysis. Instead of a mechanical stopping system the reaction is stopped by ''quenching'', the products being delivered to a recipient that stops the reaction immediately, either by instantaneous freezing or by denaturing the enzyme with a chemical denaturant or exposing the sample to a denaturing light source. As in the continuous-flow method, the time between mixing and quenching can be varied by varying the length of the tube.
 The ''pulsed quenched flow'' method introduced by
The ''pulsed quenched flow'' method introduced by Alan Fersht
Sir Alan Roy Fersht (born 21 April 1943) is a British chemist at the MRC Laboratory of Molecular Biology, Cambridge, and an Emeritus Professor in the Department of Chemistry at the University of Cambridge. He was Master of Gonville and Caius C ...
and Ross Jakes overcomes the need for a long tube. The reaction is initiated exactly as in a stopped-flow experiment, but there is a third syringe that brings about quenching a definite and preset time after the initiation.
Quenched flow has both advantages and disadvantages with respect to stopped flow. On the one hand, chemical analysis makes it clear what process is being measured, whereas it may not always be obvious what process a spectroscopic signal represents. On the other hand, quenched flow is much more laborious, as each point along the time course must be determined separately. The image at left for catalysis by nitrogenase from ''Klebsiella pneumoniae'' illustrates both of these points: the agreement in half times indicates that the absorbance at 420 nm measured the release of ''P''i, but the quenched-flow experiment required 11 data points.
References
Further reading
* * {{cite book, isbn= 978-3527330744 , title = Fundamentals of Enzyme Kinetics , edition = 4th, author = Athel Cornish-Bowden , date=2012, pages=391–396 , publisher= Wiley-Blackwell , location = Weinheim Chemical kinetics Biophysics