Stereocontrolled 1,2-addition To Carbonyl Groups on:
[Wikipedia]
[Google]
[Amazon]
 Stereocontrolled 1,2-additions to carbonyl groups (especially ketones) are an important class of reactions because they provide access to substituted alcohols, generating a new
Stereocontrolled 1,2-additions to carbonyl groups (especially ketones) are an important class of reactions because they provide access to substituted alcohols, generating a new
 Stereocontrolled 1,2-additions to carbonyl groups (especially ketones) are an important class of reactions because they provide access to substituted alcohols, generating a new
Stereocontrolled 1,2-additions to carbonyl groups (especially ketones) are an important class of reactions because they provide access to substituted alcohols, generating a new stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
in the process. Especially widespread are various reagents for stereocontrolled 1,2-hydride additions (or reductions) of ketones. A well-known method to synthesize enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
alcohols by ketone reduction is the Midland Alpine borane reduction, named after its inventor Professor M. Mark Midland. The strategy uses a chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
organoborane
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
Organoboron ...
, derived from the hydroboration of alpha-pinene
α-Pinene is an organic compound of the terpene class, one of two isomers of pinene. It is an alkene and it contains a reactive four-membered ring. It is found in the oils of many species of many coniferous trees, notably the pine. It is also ...
by 9-BBN
9-Borabicyclo .3.1onane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substr ...
, to differentiate enantiotopic In stereochemistry, topicity is the stereochemical relationship between substituents and the structure to which they are attached. Depending on the relationship, such groups can be ''heterotopic'', ''homotopic'', ''enantiotopic'', or ''diastereotop ...
faces of a ketone. Following workup with basic hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
, the product alcohols can be obtained, often with high degrees of enantioselectivity
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
. The reaction works best if one of the ketone groups has low steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
, such as an alkyne or nitrile. Another method, first developed in the 1980s, is called the Corey–Bakshi–Shibata reduction (CBS), and it features the use of an oxazaborolidine catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
along with borane as a reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth me ...
for accomplishing enantioselective ketone reductions. The CBS reduction has been used extensively by chemists en route to synthesizing a wide variety of natural products, including alkaloids, terpenoids, pheromones
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavio ...
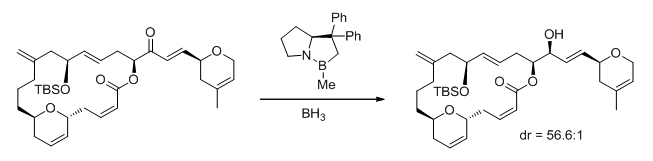
, and biotins. Fig. 1 shows an example of a diastereoselective CBS reduction being used to prepare a complex macrocyclic alcohol en route to the synthesis of 11-desmethyllaulimalide (an analog of the antitumor agent laulimalide). The authors noted that CBS reduction was much more effective than using either lithium tert-butoxyaluminum hydride or L-Selectride
L-selectride is an organoborane. It is used in organic chemistry as a reducing agent, for example in the reduction of a ketone, as part of Overman's synthesis of strychnine.
Under certain conditions, L-selectride can selectively reduce enones by ...
. The CBS catalyst, usually prepared from diphenylprolinol
Diphenylprolinol (D2PM), or (''R''/''S'')-(±)-diphenyl-2-pyrrolidinyl-methanol, is a norepinephrine-dopamine reuptake inhibitor which is used as a designer drug.
Pharmacology
The dextrorotary (''R'')-(+)-enantiomer is the more pharmacological ...
,Corey, E. J.; Bakshi, R. K.; Shibata S., J. Am. Chem. Soc. 1987, 109, 5551. often can be used in low catalyst loadings, even as low as 2%.
References
Addition reactions {{Reaction-stub