|
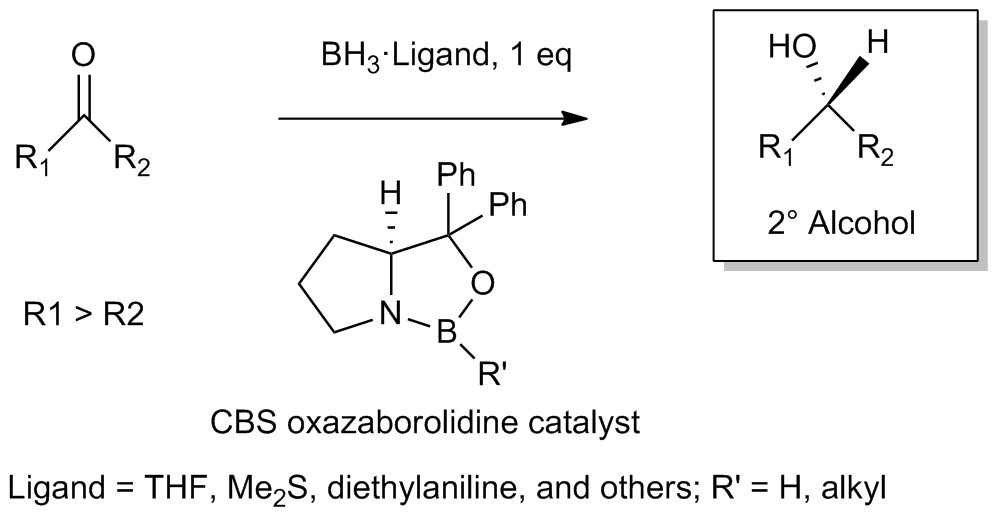
Corey–Itsuno Reduction
The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which an achiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction. History In 1981, Itsuno and coworkers first reported the use of chiral alkoxy-amine-borane complexes in reducing achiral ketones to chiral alcohols enantioselectively and in high yield. Several years later in 1987, E. J. Corey and coworkers developed the reaction between chiral amino alcohols and borane (BH3), generating oxazaborolidine products which were shown to rapidly catalyze the enantioselective reduction of achiral ketones in the presence of BH3•THF. The CBS reduction has since been utilized by organic chemists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elias James Corey
Elias James Corey (born July 12, 1928) is an American organic chemistry, organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably. Biography E.J. Corey (the surname was anglicized from Levantine Arabic ''Khoury'', meaning ''priest'') was born to Lebanese Greek Orthodox Christians, Lebanese Greek Orthodox Christian immigrants Fatima (née Hasham) and Elias Corey in Methuen, Massachusetts, north of Boston. His mother changed his name from William to "Elias" to honor his father, who died eighteen months after Corey's birth. His widowed mother, brother, two sisters, aunt and uncle all lived together in a spacious house, struggling through the Great Depression. As a young b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite. Both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used. An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The degree of selectivity is measu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kanamienamide
Kanamienamides is a complex enol ether containing enamide that is currently undergoing research in regards to its inhibitory activity towards cancer cells. The synthesis of kanamienamide consists of several chemical techniques, including CBS asymmetric reduction, Stork-Zhao- Wittig olefination, Cu-mediated amide coupling with vinyl iodide, Evans asymmetric alkylation, and ring-closing metathesis Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the ''E-' .... Kanamienamide is a natural product found in '' Moorea bouillonii'' which is a cyanobacterium. References Heterocyclic compounds with 1 ring Nitrogen heterocycles Oxygen heterocycles Amides Methoxy compounds Lactones Lactams Eleven-membered rings {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey–Link Reaction
In organic chemistry, the Corey–Link reaction is a name reaction that converts a 1,1,1-trichloro-2-keto structure into a 2-aminocarboxylic acid (an alpha amino acid) or other acyl functional group with control of the chirality at the alpha position. The reaction is named for E.J. Corey and John Link, who first reported the reaction sequence. Process The first stage of the process is the reduction of the carbonyl to give a 1,1,1-trichloro-2-hydroxy structure. The original protocol used catecholborane with a small amount of one enantiomer of CBS catalyst (a Corey–Itsuno reduction). The choice of chirality of the catalyst thus gives selectivity for one or the other stereochemistry of the alcohol, which subsequently controls the stereochemistry of the amino substituent in the ultimate product. This 2-hydroxy structure then undergoes a Jocic–Reeve reaction using azide as the nucleophile. The multistep reaction mechanism begins with deprotonation of the alcohol, followed by the o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Application Rxn
Application may refer to: Mathematics and computing * Application software, computer software designed to help the user to perform specific tasks ** Application layer, an abstraction layer that specifies protocols and interface methods used in a communications network * Function application, in mathematics and computer science Processes and documents * Application for employment, a form or forms that an individual seeking employment must fill out * College application, the process by which prospective students apply for entry into a college or university * Patent application, a document filed at a patent office to support the grant of a patent Other uses * Application (virtue), a characteristic encapsulated in diligence * Topical application, the spreading or putting of medication to body surfaces See also * * Apply In mathematics and computer science, apply is a function that applies a function to arguments. It is central to programming languages derived from lambda calcu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synlett
''Synlett'' is an international scientific journal for accounts and rapid communications of original contributions of fundamental research in synthetic organic chemistry. The impact factor of this journal is 2.419 (2017). ''Nature'' featured a brief piece by the editor-in-chief of the journal in 2017, Benjamin List Benjamin ( he, ''Bīnyāmīn''; "Son of (the) right") blue letter bible: https://www.blueletterbible.org/lexicon/h3225/kjv/wlc/0-1/ H3225 - yāmîn - Strong's Hebrew Lexicon (kjv) was the last of the two sons of Jacob and Rachel (Jacob's thir ..., where he discussed the journal's experience with the non-traditional peer review system. References Chemistry journals Thieme academic journals Publications established in 1989 {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. Structure and properties BH3 is a trigonal planar molecule with D3h symmetry. The experimentally determined B–H bond length is 119 pm. In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: :BX3 +BH4− → HBX3− + (BH3) (X=F, Cl, Br, I) :2 BH3 → B2H6 The standard enthalpy of dimerization of BH3 is estim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylaniline
Diethylaniline is the organic compound with the molecular formula (C2H5)2NC6H5. It is a colorless liquid but commercial samples are often yellow. It is a precursor to several dyes and other commercial products. Uses Its condensation with half an equivalent of benzaldehyde gives brilliant green, an analogue of the very useful malachite green. With formylbenzenedisulfonic acid it condenses to give Patent blue VE, and with hydroxybenzaldehyde followed by sulfonation one obtains Patent blue V. When treated with phosgene, one obtains Ethyl violet, an analogue of methyl violet. In organic synthesis, the complex diethylaniline·borane (DEANB) is used as a reducing agent. Safety Diethylaniline may be genotoxic because it has been found to increase the rate of sister chromatid exchange. See also * Dimethylaniline ''N'',''N''-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It consists of a tertiary amine, featuring dimethylamino group at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomeric Excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a single completely pure enantiomer has an ee of 100%. A sample with 70% of one enantiomer and 30% of the other has an ee of 40% (70% − 30%). Definition Enantiomeric excess is defined as the absolute difference between the mole fraction of each enantiomer: :\ ee = , F_R - F_S, where :\ F_R + F_S = 1 In practice, it is most often expressed as a percent enantiomeric excess. The enantiomeric excess can be determined in another way if we know the amount of each enantiomer produced. If one knows the moles of each enantiomer produced then: Enantiomeric excess is used as one of the indicators of the success of an asymmetric synthesis. For mixtures of diastereomers, there are analogous definitions and uses for diastereomeric excess an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scope Rxn2
Scope or scopes may refer to: People with the surname * Jamie Scope (born 1986), English footballer * John T. Scopes (1900–1970), central figure in the Scopes Trial regarding the teaching of evolution Arts, media, and entertainment * CinemaScope or Scope prints, anamorphic film prints * ''Scope'' (magazine), a South African men's magazine * ''The Scope (alternative weekly)'', a newspaper in St. John's, Newfoundland * ''Scope'' (Australian TV series) * ''Scope'' (Irish TV series) * ''Scope'' (album), a 1979 studio album by Buck Hill Quartet Computing * Scope (computer science), the range in which a variable can be referenced * scope (scopeArchiv), an archival information program * CDC SCOPE, a series of Control Data Corporation operating systems Concepts * Scope (logic), the range influenced by the quantification in logic * Scope (formal semantics), the natural language counterpart of logical scope * Scope (project management), the sum of all projects, products and their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroatoms
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen. Organic chemistry In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure. Typical heteroatoms are nitrogen (N), oxygen (O), sulfur (S), phosphorus (P), chlorine (Cl), bromine (Br), and iodine (I), as well as the metals lithium (Li) and magnesium (Mg). Proteins It can also be used with highly specific meanings in specialised contexts. In the description of protein structure, in particular in the Protein Data Bank file format, a heteroatom record (HETATM) describes an atom as belonging to a small molecule cofactor rather than being part of a biopolymer chain. Zeolites In the context of zeolites, the term ''heteroatom'' refers to partial isomorphous substitution of the typical framework atoms (silicon, aluminium, and phosphorus) by other elements such as beryllium, vanadium, and chromium. The goal is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |