Stem-cell niche refers to a microenvironment, within the specific anatomic location where
stem cell
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type o ...
s are found, which interacts with stem cells to regulate cell fate.
The word 'niche' can be in reference to the ''in vivo'' or ''in vitro'' stem-cell microenvironment. During embryonic development, various niche factors act on embryonic stem cells to alter gene expression, and induce their proliferation or differentiation for the development of the fetus. Within the human body, stem-cell niches maintain adult stem cells in a quiescent state, but after tissue injury, the surrounding micro-environment actively signals to stem cells to promote either self-renewal or differentiation to form new tissues. Several factors are important to regulate stem-cell characteristics within the niche: cell–cell interactions between stem cells, as well as interactions between stem cells and neighbouring differentiated cells, interactions between stem cells and adhesion molecules, extracellular matrix components, the oxygen tension, growth factors, cytokines, and the physicochemical nature of the environment including the pH, ionic strength (e.g.
Ca2+ concentration) and metabolites, like
ATP, are also important.
The stem cells and niche may induce each other during development and reciprocally signal to maintain each other during adulthood.
Scientists are studying the various components of the niche and trying to replicate the ''in vivo'' niche conditions ''in vitro''.
This is because for regenerative therapies, cell proliferation and differentiation must be controlled in flasks or plates, so that sufficient quantity of the proper cell type are produced prior to being introduced back into the patient for therapy.
Human embryonic stem cells are often grown in fibroblastic growth factor-2 containing, fetal bovine serum supplemented media. They are grown on a
feeder layer
Feeder may refer to:
Technology
* Feeder (livestock equipment)
* Feeder (beekeeping), any of several devices used in apiculture to supplement or replace natural food sources
* Feeder (casting), another name for a riser, a reservoir built into a ...
of cells, which is believed to be supportive in maintaining the pluripotent characteristics of embryonic stem cells. However, even these conditions may not truly mimic ''in vivo'' niche conditions.
Adult stem cells remain in an undifferentiated state throughout adult life. However, when they are cultured ''in vitro'', they often undergo an 'aging' process in which their morphology is changed and their proliferative capacity is decreased. It is believed that correct culturing conditions of adult stem cells needs to be improved so that adult stem cells can maintain their stemness over time.
A ''
Nature
Nature, in the broadest sense, is the physics, physical world or universe. "Nature" can refer to the phenomenon, phenomena of the physical world, and also to life in general. The study of nature is a large, if not the only, part of science. ...
'' Insight review defines niche as follows:
History
Though the concept of stem cell niche was prevailing in vertebrates, the first characterization of stem cell niche in vivo was worked out in ''
Drosophila
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species ...
'' germinal development.
The architecture of the stem-cell niche
By continuous intravital imaging in mice, researchers were able to explore the structure of the stem cell niche and to obtain the fate of individual stem cells (SCs) and their progeny over time in vivo. In particular in intestinal crypt, two distinct groups of SCs have been identified: the "border stem cells" located in the upper part of the niche at the interface with transit amplifying cells (TAs), and "central stem cells" located at the crypt base. The proliferative potential of the two groups was unequal and correlated with the cells' location (central or border). It was also shown that the two SC compartments acted in accord to maintain a constant cell population and a steady cellular turnover. A similar dependence of self-renewal potential on proximity to the niche border was reported in the context of hair follicle, in an in vivo live-imaging study.
This bi-compartmental structure of stem cell niche has been mathematically modeled to obtain the optimal architecture that leads to the maximum delay in double-hit mutant production. They found that the bi-compartmental SC architecture minimizes the rate of two-hit mutant production compared to the single SC compartment model. Moreover, the minimum probability of double-hit mutant generation corresponds to purely symmetric division of SCs with a large proliferation rate of border stem cells along with a small, but non-zero, proliferation rate of central stem cells.
Stem cell niches harboring continuously dividing cells, such as those located at the base of the
intestinal gland
In histology, an intestinal gland (also crypt of Lieberkühn and intestinal crypt) is a gland found in between villi in the intestinal epithelium lining of the small intestine and large intestine (or colon). The glands and intestinal villi are co ...
, are maintained at small population size. This presents a challenge to the maintenance of multicellular tissues, as small populations of asexually dividing individuals will accumulate deleterious mutations through
genetic drift
Genetic drift, also known as allelic drift or the Wright effect, is the change in the frequency of an existing gene variant (allele) in a population due to random chance.
Genetic drift may cause gene variants to disappear completely and there ...
and succumb to
mutational meltdown
In evolutionary genetics, mutational meltdown is a sub class of extinction vortex in which the environment and genetic predisposition mutually reinforce each other. Mutational meltdown (not to be confused with the concept of an error catastrophe) ...
.
Mathematical modeling of the intestinal gland reveals that the small population size within the stem cell niche minimizes the probability of
carcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnor ...
occurring anywhere, at the expense of gradually accumulated deleterious mutations throughout organismal lifetime—a process that contributes to tissue degradation and
aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
.
Therefore, the population size of the stem cell niche represents an
evolutionary trade-off between the probability of cancer formation and the rate of aging.
Examples
Germline
Germline stem cells (GSCs) are found in organisms that continuously produce sperm and eggs until they are sterile. These specialized stem cells reside in the GSC niche, the initial site for gamete production, which is composed of the GSCs, somatic stem cells, and other somatic cells. In particular, the GSC niche is well studied in the genetic model organism ''
Drosophila melanogaster
''Drosophila melanogaster'' is a species of fly (the taxonomic order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly" or "pomace fly". Starting with Ch ...
'' and has provided an extensive understanding of the molecular basis of stem cell regulation.
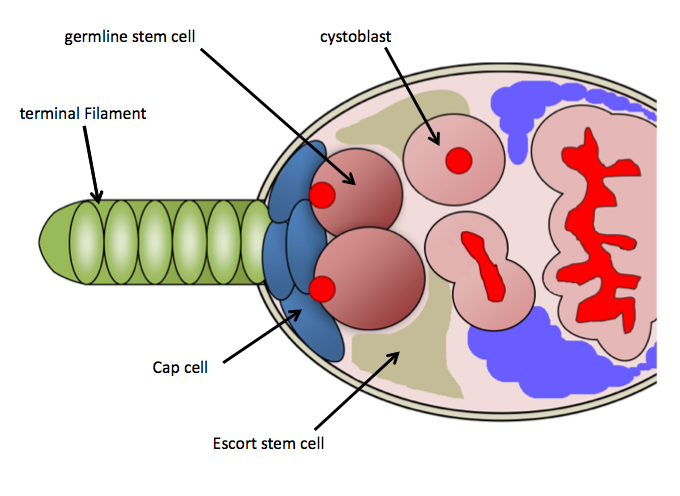
GSC niche in ''Drosophila'' ovaries
In ''Drosophila melanogaster'', the GSC niche resides in the anterior-most region of each
ovariole
An ovariole is a tubular component of the insect ovary, and the basic unit of egg production. Each ovariole is composed of a germarium (the germline stem cell niche) at the anterior tip, a set of developing oocytes contained within follicles, and ...
, known as the germarium. The GSC niche consists of necessary somatic cells-terminal filament cells, cap cells, escort cells, and other stem cells which function to maintain the GSCs.
The GSC niche holds on average 2–3 GSCs, which are directly attached to somatic cap cells and Escort stem cells, which send maintenance signals directly to the GSCs.
GSCs are easily identified through histological staining against
vasa protein (to identify germ cells) and 1B1 protein (to outline cell structures and a germline specific fusome structure). Their physical attachment to the cap cells is necessary for their maintenance and activity.
A GSC will divide asymmetrically to produce one daughter cystoblast, which then undergoes 4 rounds of incomplete mitosis as it progresses down the ovariole (through the process of
oogenesis
Oogenesis, ovogenesis, or oögenesis is the differentiation of the ovum (egg cell) into a cell competent to further develop when fertilized. It is developed from the primary oocyte by maturation. Oogenesis is initiated in the embryonic stage.
O ...
) eventually emerging as a mature egg chamber; the fusome found in the GSCs functions in cyst formation and may regulate asymmetrical cell divisions of the GSCs.
Because of the abundant genetic tools available for use in ''Drosophila melanogaster'' and the ease of detecting GSCs through
histological
Histology,
also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures vis ...
stainings, researchers have uncovered several molecular pathways controlling GSC maintenance and activity.
Molecular mechanisms of GSC maintenance and activity
= Local signals
=
The Bone Morphogenetic Protein (BMP) ligands
Decapentaplegic
Decapentaplegic (Dpp) is a key morphogen involved in the development of the fruit fly ''Drosophila melanogaster'' and is the first validated secreted morphogen. It is known to be necessary for the correct patterning and development of the early '' ...
(Dpp) and Glass-bottom-boat (Gbb) ligand are directly signalled to the GSCs, and are essential for GSC maintenance and self-renewal.
BMP signalling in the niche functions to directly repress expression of ''Bag-of-marbles'' (''Bam'') in GSCs, which is up-regulated in developing cystoblast cells.
Loss of function of ''dpp'' in the niche results in de-repression of Bam in GSCs, resulting in rapid differentiation of the GSCs.
Along with BMP signalling, cap cells also signal other molecules to GSCs: Yb and
Piwi
Piwi (or PIWI) genes were identified as gene regulation, regulatory proteins responsible for stem cell and germ cell cell differentiation, differentiation. Piwi is an abbreviation of P element, P-element Induced WImpy testis in ''Drosophila ...
. Both of these molecules are required non-autonomously to the GSCs for proliferation-''piwi'' is also required autonomously in the GSCs for proliferation.
In the germarium, BMP signaling has a short-range effect, therefore the physical attachment of GSCs to cap cells is important for maintenance and activity.
=Physical attachment of GSCs to cap cells
=
The GSCs are physically attached to the
cap cells by Drosophila
E-cadherin
Cadherin-1 or Epithelial cadherin (E-cadherin), (not to be confused with the APC/C activator protein CDH1) is a protein that in humans is encoded by the ''CDH1'' gene. Mutations are correlated with gastric, breast, colorectal, thyroid, and ovarian ...
(DE-cadherin) adherens junctions and if this physical attachment is lost GSCs will differentiate and lose their identity as a stem cell.
The gene encoding DE-cadherin, ''shotgun'' (''shg''), and a gene encoding Beta-catenin ortholog, ''armadillo'', control this physical attachment.
A GTPase molecule, rab11, is involved in cell trafficking of DE-cadherins. Knocking out ''rab11'' in GSCs results in detachment of GSCs from the cap cells and premature differentiation of GSCs.
Additionally, ''zero population growth'' (''zpg''), encoding a germline-specific
gap junction
Gap junctions are specialized intercellular connections between a multitude of animal cell-types. They directly connect the cytoplasm of two cells, which allows various molecules, ions and electrical impulses to directly pass through a regulate ...
is required for germ cell differentiation.
=Systemic signals regulating GSCs
=
Both diet and insulin-like signaling directly control GSC proliferation in ''Drosophila melanogaster''. Increasing levels of ''Drosophila'' insulin-like peptide (DILP) through diet results in increased GSC proliferation.
Up-regulation of DILPs in aged GSCs and their niche results in increased maintenance and proliferation.
It has also been shown that DILPs regulate cap cell quantities and regulate the physical attachment of GSCs to cap cells.
=Renewal mechanisms
=
There are two possible mechanisms for stem cell renewal, symmetrical GSC division or de-differentiation of cystoblasts. Normally, GSCs will divide asymmetrically to produce one daughter cystoblast, but it has been proposed that symmetrical division could result in the two daughter cells remaining GSCs.
If GSCs are ablated to create an empty niche and the cap cells are still present and sending maintenance signals, differentiated cystoblasts can be recruited to the niche and de-differentiate into functional GSCs.
Stem cell aging
As the ''Drosophila'' female ages, the stem cell niche undergoes age-dependent loss of GSC presence and activity. These losses are thought to be caused in part by degradation of the important signaling factors from the niche that maintains GSCs and their activity. Progressive decline in GSC activity contributes to the observed reduction in fecundity of ''Drosophila melanogaster'' at old age; this decline in GSC activity can be partially attributed to a reduction of signaling pathway activity in the GSC niche.
It has been found that there is a reduction in Dpp and Gbb signaling through aging. In addition to a reduction in niche signaling pathway activity, GSCs age cell-autonomously. In addition to studying the decline of signals coming from the niche, GSCs age intrinsically; there is age-dependent reduction of adhesion of GSCs to the cap cells and there is accumulation of Reactive Oxygen species (ROS) resulting in cellular damage which contributes to GSC aging. There is an observed reduction in the number of cap cells and the physical attachment of GSCs to cap cells through aging. ''Shg'' is expressed at significantly lower levels in an old GSC niche in comparison to a young one.
GSC niche in ''Drosophila'' testes
Males of ''Drosophila melanogaster'' each have two testes – long, tubular, coiled structures – and at the anterior most tip of each lies the GSC niche. The testis GSC niche is built around a population of non-mitotic hub cells (a.k.a. niche cells), to which two populations of stem cells adhere: the GSCs and the somatic stem cells (SSCs, a.k.a. somatic cyst stem cells/cyst stem cells). Each GSC is enclosed by a pair of SSCs, though each stem cell type is still in contact with the hub cells. In this way, the stem cell niche consists of these three cell types, as not only do the hub cells regulate GSC and SSC behaviour, but the stem cells also regulate the activity of each other. The Drosophila testis GSC niche has proven a valuable model system for examining a wide range of cellular processes and signalling pathways.
Outside the testis GSC niche
The process of spermatogenesis begins when the GSCs divide asymmetrically, producing a GSC that maintains hub contact, and a gonialblast that exits the niche. The SSCs divide with their GSC partner, and their non-mitotic progeny, the somatic cyst cells (SCCs, a.k.a. cyst cells) will enclose the gonialblast. The gonialblast then undergoes four rounds of synchronous, transit-amplifying divisions with incomplete cytokinesis to produce a sixteen-cell spermatogonial cyst. This spermatogonial cyst then differentiates and grows into a spermatocyte, which will eventually undergo meiosis and produce sperm.
Molecular signalling in the testis GSC niche
The two main molecular signalling pathways regulating stem cell behaviour in the testis GSC niche are the Jak-STAT and BMP signalling pathways. Jak-STAT signalling originates in the hub cells, where the ligand Upd is secreted to the GSCs and SSCs. This leads to activation of the ''Drosophila'' STAT, Stat92E, a transcription factor which effects GSC adhesion to the hub cells, and SSC self-renewal via Zfh-1. Jak-STAT signalling also influences the activation of BMP signalling, via the ligands Dpp and Gbb. These ligands are secreted into the GSCs from the SSCs and hub cells, activate BMP signalling, and suppress the expression of Bam, a differentiation factor. Outside of the niche, gonialblasts no longer receive BMP ligands, and are free to begin their differentiation program. Other important signalling pathways include the MAPK and Hedgehog, which regulate germline enclosure and somatic cell self-renewal, respectively.
GSC niche in mouse testes
The murine GSC niche in males, also called spermatogonial stem cell (SSC) niche, is located in the basal region of seminiferous tubules in the testes. The seminiferous epithelium is composed of sertoli cells that are in contact with the basement membrane of the tubules, which separates the sertoli cells from the interstitial tissue below. This interstitial tissue comprises Leydig cells, macrophages, mesenchymal cells, capillary networks, and nerves.
During development, primordial germ cells migrate into the seminiferous tubules and downward towards the basement membrane whilst remaining attached to the sertoli cells where they will subsequently differentiate into SSCs, also referred to as Asingle spermatogonia.
These SSCs can either self-renew or commit to differentiating into spermatozoa upon the proliferation of Asingle into Apaired spermatogonia. The 2 cells of Apaired spermatogonia remain attached by intercellular bridges and subsequently divide into Aaligned spermatogonia, which is made up of 4–16 connected cells. Aaligned spermatogonia then undergo meiosis I to form spermatocytes and meiosis II to form spermatids which will mature into spermatozoa.
This differentiation occurs along the longitudinal axis of sertoli cells, from the basement membrane to the apical lumen of the seminiferous tubules. However, sertoli cells form tight junctions that separate SSCs and spermatogonia in contact with the basement membrane from the spermatocytes and spermatids to create a basal and an adluminal compartment, whereby differentiating spermatocytes must traverse the tight junctions.
These tight junctions form the blood testis barrier (BTB) and have been suggested to play a role in isolating differentiated cells in the adluminal compartment from secreted factors by the interstitial tissue and vasculature neighboring the basal compartment.
Molecular mechanisms of SSC maintenance and activity
= Physical cues
=
The basement membrane of the seminiferous tubule is a modified form of extracellular matrix composed of fibronectin, collagens, and laminin.
β1- integrin is expressed on the surface of SSCs and is involved in their adhesion to the laminin component of the basement membrane although other adhesion molecules are likely also implicated in the attachment of SSCs to the basement membrane.
E cadherin expression on SSCs in mice, unlike in ''Drosophila'', have been shown to be dispensable as the transplantation of cultured SSCs lacking E-cadherin are able to colonize host seminiferous tubules and undergo spermatogenesis.
In addition the blood testis barrier provides architectural support and is composed of tight junction components such as occludins, claudins and zonula occludens (ZOs) which show dynamic expression during spermatogenesis.
For example, claudin 11 has been shown to be a necessary component of these tight junctions as mice lacking this gene have a defective blood testis barrier and do not produce mature spermatozoa.
=Molecular signals regulating SSC renewal
=
GDNF (Glial cell-derived neurotrophic factor) is known to stimulate self-renewal of SSCs and is secreted by the sertoli cells under the influence of gonadotropin FSH. GDNF is a related member of the TGFβ superfamily of growth factors and when overexpressed in mice, an increase in undifferentiated spermatogonia was observed which led to the formation of germ tumours.
In corroboration for its role as a renewal factor, heterozygous knockout male mice for GDNF show decreased spermatogenesis that eventually leads to infertility.
In addition the supplementation of GDNF has been shown to extend the expansion of mouse SSCs in culture. However, the GDNF receptor c-RET and co-receptor GFRa1 are not solely expressed on the SSCs but also on Apaired and Aaligned, therefore showing that GDNF is a renewal factor for Asingle to Aaligned in general rather than being specific to the Asingle SSC population. FGF2 (Fibroblast growth factor −2), secreted by sertoli cells, has also been shown to influence the renewal of SSCs and undifferentiated spermatogonia in a similar manner to GDNF.
Although sertoli cells appear to play a major role in renewal, it expresses receptors for testosterone that is secreted by Leydig cells whereas germ cells do not contain this receptor- thus alluding to an important role of Leydig cells upstream in mediating renewal. Leydig cells also produce CSF 1 (Colony stimulating factor −1) for which SSCs strongly express the receptor CSF1R.
When CSF 1 was added in culture with GDNF and FGF2 no further increase in proliferation was observed, however, the longer the germ cells remained in culture with CSF-1 the greater the SSC density observed when these germ cells were transplanted into host seminiferous tubules. This showed CSF 1 to be a specific renewal factor that tilts the SSCs towards renewal over differentiation, rather than affecting proliferation of SSCs and spermatogonia. GDNF, FGF 2 and CSF 1 have also been shown to influence self-renewal of stem cells in other mammalian tissues.
Plzf (Promyelocytic leukaemia zinc finger) has also been implicated in regulating SSC self-renewal and is expressed by Asingle, Apaired and Aaligned spermatogonia. Plzf directly inhibits the transcription of a receptor, c-kit, in these early spermatogonia. However, its absence in late spermatogonia permits c-kit expression, which is subsequently activated by its ligand SCF (stem cell factor) secreted by sertoli cells, resulting in further differentiation. Also, the addition of BMP4 and Activin-A have shown to reduce self-renewal of SSCs in culture and increase stem cell differentiation, with BMP4 shown to increase the expression of c-kit.
= Aging of the SSC niche
=
Prolonged spermatogenesis relies on the maintenance of SSCs, however, this maintenance declines with age and leads to infertility. Mice between 12 and 14 months of age show decreased testis weight, reduced spermatogenesis and SSC content. Although stem cells are regarded as having the potential to infinitely replicate in vitro, factors provided by the niche are crucial in vivo. Indeed, serial transplantation of SSCs from male mice of different ages into young mice 3 months of age, whose endogenous spermatogenesis had been ablated, was used to estimate stem cell content given that each stem cell would generate a colony of spermatogenesis.
The results of this experiment showed that transplanted SSCs could be maintained far longer than their replicative lifespan for their age. In addition, a study also showed that SSCs from young fertile mice could not be maintained nor undergo spermatogenesis when transplanted into testes of old, infertile mice. Together, these results points towards a deterioration of the SSC niche itself with aging rather than the loss of intrinsic factors in the SSC.
Vertebrate adult stem cell niches
Hematopoietic stem cell niche
Vertebrate
hematopoietic stem cell
Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the very first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within t ...
s niche in the
bone marrow
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis). It is composed of hematopoietic ce ...
is formed by cells subendosteal osteoblasts,
sinusoidal endothelial cells
A liver sinusoid is a type of capillary known as a sinusoidal capillary, discontinuous capillary or sinusoid, that is similar to a fenestrated capillary, having discontinuous endothelium that serves as a location for mixing of the oxygen-rich blo ...
and bone marrow stromal (also sometimes called reticular) cells which includes a mix of
fibroblast
A fibroblast is a type of cell (biology), biological cell that synthesizes the extracellular matrix and collagen, produces the structural framework (Stroma (tissue), stroma) for animal Tissue (biology), tissues, and plays a critical role in wound ...
oid,
monocytic and
adipocytic cells (which comprise
marrow adipose tissue
Bone marrow adipose tissue (BMAT), sometimes referred to as marrow adipose tissue (MAT), is a type of adipose tissue, fat deposit in bone marrow. It increases in states of low bone density -osteoporosis, anorexia nervosa/ Calorie restriction, cal ...
).
[
]
Hair follicle stem cell niche
The hair follicle stem cell niche is one of the more closely studied niches thanks to its relative accessibility and role in important diseases such as melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ( ...
. The bulge area at the junction of arrector pili muscle
The arrector pili muscles, also known as hair erector muscles, are small muscles attached to hair follicles in mammals. Contraction of these muscles causes the hairs to stand on end, known colloquially as goose bumps (piloerection).
Structure
...
to the hair follicle sheath has been shown to host the skin stem cells which can contribute to all epithelial
Epithelium or epithelial tissue is one of the four basic types of animal tissue, along with connective tissue, muscle tissue and nervous tissue. It is a thin, continuous, protective layer of compactly packed cells with a little intercellula ...
skin layers. There cells are maintained by signaling in concert with niche cell
Stem-cell niche refers to a microenvironment, within the specific anatomic location where stem cells are found, which interacts with stem cells to regulate cell fate. The word 'niche' can be in reference to the ''in vivo'' or ''in vitro'' stem-cell ...
s – signals include paracrine Paracrine signaling is a form of cell signaling, a type of cellular communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour of those cells. Signaling molecules known as paracrine factors diffuse ove ...
(e.g. sonic hedgehog
Sonic hedgehog protein (SHH) is encoded for by the ''SHH'' gene. The protein is named after the character ''Sonic the Hedgehog''.
This signaling molecule is key in regulating embryonic morphogenesis in all animals. SHH controls organogenesis and ...
), autocrine Autocrine signaling is a form of cell signaling in which a cell secretes a hormone or chemical messenger (called the autocrine agent) that binds to autocrine receptors on that same cell, leading to changes in the cell. This can be contrasted with pa ...
and juxtacrine
In biology, juxtacrine signalling (or contact-dependent signalling) is a type of cell–cell or cell–extracellular matrix signalling in multicellular organisms that requires close contact. In this type of signalling, a ligand on one surface bin ...
signals. The bulge region of the hair follicle relies on these signals to maintain the stemness of the cells. Fate mapping
Fate mapping is a method used in developmental biology to study the embryonic origin of various adult tissues and structures. The "fate" of each cell or group of cells is mapped onto the embryo, showing which parts of the embryo will develop into ...
or cell lineage tracing has shown that Keratin 15
Keratin 15 is a protein that in humans is encoded by the ''KRT15'' gene. It has also been referred to as cytokeratin 15, K1CO and KRTB.
Keratin 15 is a type I cytokeratin Type I keratins (or Type I cytokeratins) are cytokeratins that constitute ...
positive stem cells' progeny participate in all epithelial lineages. The follicle undergoes cyclic regeneration in which these stem cells migrate to various regions and differentiate into the appropriate epithelial cell type. Some important signals in the hair follicle stem cell niche produced by the mesenchymal dermal papilla or the bulge include BMP, TGF-β
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other sign ...
and Fibroblast growth factor
Fibroblast growth factors (FGF) are a family of cell signalling proteins produced by macrophages; they are involved in a wide variety of processes, most notably as crucial elements for normal development in animal cells. Any irregularities in the ...
(FGF) ligands and Wnt inhibitors. While, Wnt signaling pathways and β-catenin are important for stem cell maintenance, over-expression of β-catenin
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the ''CTNNB1'' gene.
Beta-catenin is a dual function protein, involved in regulation and coordination of cell–cell adhesion and gene transcriptio ...
in hair follicles induces improper hair growth. Therefore, these signals such as Wnt inhibitors produced by surrounding cells are important to maintain and facilitate the stem cell niche.
Intestinal stem cell niche
Intestinal organoid
An organoid is a miniaturized and simplified version of an Organ (anatomy), organ produced in vitro in three dimensions that shows realistic micro-anatomy. They are derived from one or a few Cell (biology), cells from a Tissue (biology), tissue, ...
s have been used to study intestinal stem cell niches. An intestinal organoid culture can be used to indirectly assess the effect of the manipulation on the stem cells through assessing the organoid's survival and growth. Research using intestinal organoids have demonstrated that the survival of intestinal stem cells is improved by the presence of neurons and fibroblasts, and through the administration of IL-22.
Cardiovascular stem cell niche
Cardiovascular stem cell niches can be found within the right ventricular free wall, atria and outflow tracks of the heart. They are composed of Isl1+/Flk1+ cardiac progenitor cells (CPCs) that are localized into discrete clusters within a ColIV and laminin extracellular matrix (ECM). ColI and fibronectin are predominantly found outside the CPC clusters within the myocardium. Immunohistochemical staining has been used to demonstrate that differentiating CPCs, which migrate away from the progenitor clusters and into the ColI and fibronectin ECM surrounding the niche, down-regulate Isl1 while up-regulating mature cardiac markers such as troponin C.
Neural stem cell niche
Neural stem cell niches are divided in two : the Subependymal zone (SEZ) and the Subgranular zone
The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.
Structure
The subgranular zone is a narrow layer of cell ...
(SGZ).
The SEZ is a thin area beneath the ependymal cell layer that contains three types of neural stem cells : infrequently dividing neural stem cells (NSCs), rapidly dividing transit amplifying precursors (TaPs) and neuroblasts (NBs). The SEZ extracellular matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide stru ...
(ECM) has significant differences in composition compared to surrounding tissues. Recently, it was described that progenitor cells, NSCs, TaPs and NBs were attached to ECM structures called Fractones. These structures are rich in laminin, collagen and heparan sulfate proteoglycans. Other ECM molecules, such as tenascin-C, MMPs and different proteoglycans are also implicated in the neural stem cell niche.
Cancer stem cell niche
Cancer tissue is morphologically heterogenous, not only due to the variety of cell types present, endothelial, fibroblast and various immune cells, but cancer cells themselves are not a homogenous population either.
In accordance with the hierarchy model of tumours, the cancer stem cell
Cancer stem cells (CSCs) are cancer cells (found within tumors or hematological cancers) that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. ...
s (CSC) are maintained by biochemical and physical contextual signals emanating from the microenvironment, called the cancer stem cell niche.embryonic stem cell
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre- implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consi ...
(ESC), Adult Stem Cell ASC) in function (maintaining of self-renewal, undifferentiated state and ability to differentiate) and in signalling pathways (Activin/Noda, Akt/PTEN, JAK/STAT, PI3-K, TGF-β, Wnt and BMP).
Hypoxia
Hypoxic
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the t ...
condition in stem cell niches (ESC, ASC or CSC) is necessary for maintaining stem cells in an undifferentiated state and also for minimizing DNA damage via oxidation. The maintaining of the hypoxic state is under control of Hypoxia-Inducible transcription Factors (HIFs).
Hypoxia in the CSC niche
Hypoxia plays an important role in the regulation of cancer stem cell niches and EMT through the promotion of HIFs. These HIFs help maintain cancer stem cell niches by regulating important stemness genes such as Oct4
Oct-4 (octamer-binding transcription factor 4), also known as POU5F1 (POU domain, class 5, transcription factor 1), is a protein that in humans is encoded by the ''POU5F1'' gene. Oct-4 is a homeodomain transcription factor of the POU family. I ...
, Nanog, SOX2, Klf4, and cMyc. HIFs also regulate important tumor suppressor genes such as p53
p53, also known as Tumor protein P53, cellular tumor antigen p53 (UniProt name), or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins (originally thought to be, and often s ...
and genes that promote metastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, ...
. Although HIFs increase the survival of cells by decreasing the effects of oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily Detoxification, detoxify the reactive intermediates or to repair the resulting damage. Disturbances ...
, they have also been shown to decrease factors such as RAD51
DNA repair protein RAD51 homolog 1 is a protein encoded by the gene ''RAD51''. The enzyme encoded by this gene is a member of the RAD51 protein family which assists in repair of DNA double strand breaks. RAD51 family members are homologous to th ...
and H2AX that maintain genomic stability. In the hypoxic condition there is an increase of intracellular Reactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS) which also promote CSCs survival via stress response. ROS stabilizes HIF-1α which promotes the Met proto-oncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels. , which drives metastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, ...
or motogenic escape in melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ( ...
cells. All of these factors contribute to a cancer stem cell phenotype which is why it is often referred to as a hypoxic stem cell niche. Hypoxic environments are often found in tumors where the cells are dividing faster that angiogenesis
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature by processes of sprouting and splitting ...
can occur. It is important to study hypoxia as an aspect of cancer because hypoxic environments have been shown to be resistant to radiation therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radia ...
. Radiation has been shown to increase the amounts of HIF-1
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in available oxygen in the cellular environment, or hypoxia. They are only present in parahoxozoan animals.
Discovery
The HIF transcriptional complex w ...
. EMT induction by hypoxia though interactions between HIF-1α and ROS is crucial for metastasis in cancers such as melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ( ...
. It has been found that many genes associated with melanoma are regulated by hypoxia such as MXI1, FN1, and NME1.
Epithelial–mesenchymal transition
Epithelial–mesenchymal transition
The epithelial–mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal ...
is a morphogenetic process, normally occurs in embryogenesis that is "hijacked" by cancer stem cells by detaching from their primary place and migrating to another one. The dissemination is followed by reverse transition so-called Epithelial-Mesenchymal Transition (EMT). This process is regulated by CSCs microenvironment via the same signalling pathways as in embryogenesis using the growth factors (TGF-β
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other sign ...
, PDGF
Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood v ...
, EGF), cytokine IL-8 and extracellular matrix components. These growth factors' interactions through intracellular signal transducers like β-catenin
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the ''CTNNB1'' gene.
Beta-catenin is a dual function protein, involved in regulation and coordination of cell–cell adhesion and gene transcriptio ...
has been shown to induce metastatic potential. A characteristic of EMT is loss of the epithelial markers (E-cadherin, cytokeratins, claudin, occluding, desmoglein, desmocolin) and gain of mesenchymal markers (N-cadherin, vimentin, fibronectin).
Inflammation
The EMT and cancer progression can be triggered also by chronic inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
. The main roles have molecules (IL-6, IL-8, TNF-α, NFκB, TGF-β, HIF-1α) which can regulate both processes through regulation of downstream signalling that overlapping between EMT and inflammation.
Angiogenesis
Hypoxia is a main stimulant for angiogenesis
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature by processes of sprouting and splitting ...
, with HIF-1α being the primary mediator. Angiogenesis induced by hypoxic conditions is called an "Angiogenic switch". HIF-1 promotes expression of several angiogenic factors: Vascular Endothelial Growth Factor (VEGF), basic Fibroblast Growth Factor (bFGF), Placenta-Like Growth Factor (PLGF), Platelet-Derived Growth Factor (PDGF) and Epidermal Growth Factor. But there is evidence that the expression of angiogenic agens by cancer cells can also be HIF-1 independent. It seems that there is an important role of Ras protein, and that intracellular levels of calcium regulate the expression of angiogenic genes in response to hypoxia.
Injury-induced
During injury, support cells are able to activate a program for repair, recapitulating aspects of development in the area of damage. These areas become permissive for stem cell renewal, migration and differentiation. For instance in the CNS, injury is able to activate a developmental program in astrocytes that allow them to express molecules that support stem cells such as chemokines i.e. SDF-1 and morphogens such as sonic hedgehog.
Extracellular Matrix Mimicking Strategies For Stem Cell Niche
It is evident that biophysio-chemical characteristics of ECM such as composition, shape, topography, stiffness, and mechanical strength can control the stem cell behavior. These ECM factors are equally important when stem cells are grown in vitro. Given a choice between niche cell-stem cell interaction and ECM-stem cell interaction, mimicking ECM is preferred as that can be precisely controlled by scaffold fabrication techniques, processing parameters or post-fabrication modifications. In order to mimic, it is essential to understand natural properties of ECM and their role in stem cell fate processes. Various studies involving different types of scaffolds that regulate stem cells fate by mimicking these ECM properties have been done.
References
{{Reflist
Stem cells
Habitats
