Spirocyclic on:
[Wikipedia]
[Google]
[Amazon]
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like
an
respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a

same access date. The Greek transcription, σπεῖρα, reflects the use of this cognate as one ancient Greek term to refer to a coil or related fold, see is a chemical compound, typically an organic compound, that presents a twisted structure of two or more rings (a ring system), in which 2 or 3 rings are linked together by one common atom, Note, the article co-authors, the Working Party of the IUPAC (1992-1998), were P. M. Giles, Jr., E. W. Godly, K.-H. Hellwich, A. K. Ikizler, M. V. Kisakürek, A. D. McNaught, G. P. Moss, J. Nyitrai, W. H. Powell, O. Weissbach, and A. Yerin. ''Also available online at'' ''Also available in German, with et al. indicating the same working party, at'' examples of which are shown at right.
same access date. * Examples of spiro natural products and their synthesis: * * The IUPAC documents on naming of spiro compounds: The full author (Working Party) list and a link to a German translation are provided in a corresponding footnote. ''Also available online at'' , same access date.
biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
that have no connecting atoms, from ''fused ring compounds'' like decalin having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan
respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a
diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
with a cyclic ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
. The common atom that connects the two (or sometimes three) rings is called the ''spiro atom''; in carbocyclic spiro compounds like spiro .5ndecane (see image at right), the spiro-atom is a quaternary carbon, and as the ''-ane'' ending implies, these are the types of molecules to which the name ''spirane'' was first applied (though it is now used general of all spiro compounds). Likewise, a tetravalent neutral silicon or positively charged quaternary nitrogen atom (ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
cation) can be the spiro center in these compounds, and many of these have been prepared and described. The 2-3 rings being joined are most often different in nature, though they, on occasion, be identical .5ndecane, just shown, and .g., spiro .5ndecane, just shown, and spiropentadiene, at right">spiropentadiene">.g., spiro .5ndecane, just shown, and spiropentadiene, at right Although sketches of organic structures makes spiro compounds appear planar, they are not; for instance, a spiro compound with a pair of three-membered cyclopropene rings connected in spiro fashion (image below) has been given the popular misnomer of being a bow tie structure, when it is not flat or planar like a bow tie. This can be stated another way, saying that the best-fit planes
Plane(s) most often refers to:
* Aero- or airplane, a powered, fixed-wing aircraft
* Plane (geometry), a flat, 2-dimensional surface
Plane or planes may also refer to:
Biology
* Plane (tree) or ''Platanus'', wetland native plant
* ''Planes' ...
to each ring are often perpendicular or are otherwise non-coplanar to one another.
Spiro compounds are present throughout the natural world, some cases of which have been exploited to provide tool compounds for biomedical study and to serve as scaffolds for the design of therapeutic agents with novel shapes. As well, the spiro motif is present in various practical compound types (such as dyes), as well as in a wide variety of oligo- and polymeric materials designs, for the unique shapes and properties the spiro center imparts, e.g., in the design of electronically active materials in particular. In both cases, the presence of the spiro center, often with four distinct groups attached, and with its unique aspects of chirality, adds unique challenges to the chemical synthesis of each compound type.
Carbocyclic spiro compounds
Bicyclic ring structures in organic chemistry that have two fully carbocyclic (all carbon) rings connected through just one atom are present both in natural products, as well as in esoteric targets of chemical synthesis. The two carbocycles can be different in nature, or identical. In common targets derived from natural products, they are essentially always different. In esoteric targets, such as highly strained hydrocarbons like spiropentadiene, shown here, the rings can be identical. The atom connecting the two rings is called the ''spiro-atom''; in carbocyclic spiro compounds, the spiro-atom is a quaternary carbon. The 11-carbon bicyclic structure shown above, spiro .5ndecane, is also a fully carbocyclic spiro compound. While the presentation of this structure makes it appear fully planar, it is not. The best-fit planes to each six-atom ring above is near to perpendicular, and the best-fit planes to rings of spiro compounds are likewise generally non-coplanar. For instance, the structure of faux bow tie spiropentadiene, shown above, makes clear that theplanes
Plane(s) most often refers to:
* Aero- or airplane, a powered, fixed-wing aircraft
* Plane (geometry), a flat, 2-dimensional surface
Plane or planes may also refer to:
Biology
* Plane (tree) or ''Platanus'', wetland native plant
* ''Planes' ...
that are defined by the atoms of each ring—i.e., the best-fit plane of each cyclopropene—are orthogonal (perpendicular) to one another.
Heterocyclic spiro compounds
Spiro compounds are considered heterocyclic if the spiro atom or any atom in either ring are not carbon atoms. Cases include the presence of a spiro heteroatom such silicon and nitrogen (but also other Group IVA 4and other atom types) connecting the rings that have been observed or are under theoretical study; moreover, there are also many cases where one or more heteroatoms appear in one or more of the rings that are joined at a carbon spiro atom (e.g., where 1 oxygen spironolactones and 2 oxygen/2 sulfur ketals/thioketals are very common). A common case is the presence of two atoms that are not carbon in one of the rings, with those two rings both attached to the spiro atom; indeed, often the earliest exposure of a chemist in training to a spiro compound is to a heterocyclic form, the ketal (acetal) formed in the protection of ketones bydiol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
s and dithiols. An example of this is shown above, in the synthesis of the acetal 1,4-dioxaspiro .5ecane from cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexan ...
and ethanediol. In this case, because the four atoms attached to the spiro atom are not all carbons, the spiro atom is not a quaternary carbon. A further example of an acetal formed from a cyclic ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
, except with a dithiol, is the spiro compound spirapril
Spirapril, sold under the brand name Renormax among others, is an ACE inhibitor antihypertensive drug used to treat hypertension. It belongs to dicarboxy group of ACE inhibitors.
It was patented in 1980 and approved for medical use in 1995.
Ch ...
, which has a five-membered ring formed from 1,2-ethanedithiol
Ethane-1,2-dithiol, also known as EDT, is a colorless liquid with the formula C H( SH). It has a very characteristic odor which is compared by many people to rotten cabbage. It is a common building block in organic synthesis and an excellent liga ...
. Again, while the rings could be identical, in the heterocyclic case they are, again, almost always non-identical. Once again, the best-fit planes to each ring are generally non-coplanar to one another (i.e., the rings are not coplanar, despite appearing so in images).
Polyspiro compounds
A polyspiro compound is connected by two or more spiroatoms making up three or more rings.Nomenclature
Nomenclature for spiro compounds was first discussed by Adolf von Baeyer in 1900. The prefix ''spiro'' denotes two rings with a spiro junction. The main method of systematic nomenclature is to follow with square brackets containing the number of atoms in the smaller ring then the number of atoms in the larger ring, separated by a period, in each case excluding the spiroatom (the atom by which the two rings are bonded) itself. Position-numbering starts with an atom of the smaller ring adjacent to the spiroatom around the atoms of that ring, then the spiroatom itself, then around the atoms of the larger ring. For example, compound A in the image is called ''1-bromo-3-chlorospiro .5ecan-7-ol'', and compound B is called ''1-bromo-3-chlorospiro .6ecan-7-ol''.Chirality
Spiranes can be chiral, in three distinct ways. First, while nevertheless appearing to be twisted, they yet may have a chiral center making them analogous to any simplechiral compound
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are d ...
, and second, while again appearing twisted, the specific location of substituents, as with alkylidenecycloalkanes, may make a spiro compound display ''central chirality'' (rather than axial chirality resulting from the twist); third, the substituents of the rings of the spiro compound may be such that the only reason they are chiral arises solely from the twist of their rings, e.g., in the simplest bicyclic case, where two structurally identical rings are attached via their spiro atom, resulting in a twisted presentation of the two rings. Hence, in the third case, the lack of planarity described above gives rise to what is termed axial chirality
Axial may refer to:
* one of the anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring to an axial bond
* a type of modal frame, in music
* ...
in otherwise identical isomeric pair of spiro compounds, because they differ only in the right- ''versus'' left-handed "twist" of structurally identical rings (as seen in allenes, sterically hindered biaryl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
s, and alkylidenecycloalkanes as well). Assignment of absolute configuration of spiro compounds has been challenging, but a number of each type have been unequivocally assigned.
Some spiro compounds exhibit axial chirality
Axial may refer to:
* one of the anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring to an axial bond
* a type of modal frame, in music
* ...
. Spiroatoms can be the origin of chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
even when they lack the required four different substituents normally observed in chirality. When two rings are identical the priority is determined by a slight modification of the CIP system
CIP may refer to:
Business and finance
* Commercially Important Person
* Construction in progress, a balance sheet assets item
* Continual improvement process
* "Carriage and Insurance Paid to" Incoterms
* Customer Identification Program, in US ...
assigning a higher priority to one ring extension and a lower priority to an extension in the other ring. When rings are dissimilar the regular rules apply.
Preparation
Spiro compounds present unique preparative challenges, whether each ring contributing to its structure is unique or identical, or whether they are carbocyclic or heterocyclic—owing to the practical implications of tetra-functionalizing the central spiro atom (often with four different groups), and of the unique aspects ofchirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
that apply to these compounds.
Specific methods
Some spiro compounds can be synthesized using the Pinacol-pinacolone rearrangement; for example, spiro .5ecane (final compound in following two line scheme) can be synthesized from symmetric 1,2-diols of the sort shown below .g., this route's starting material, (1,1′-bicyclopentyl)-1,1′-diol Initially, one of the carbinol moieties is protonated, allowing water to leave, and yielding the correspondingcarbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
(second structure, first row); this intermediate then undergoes a bond migration, resulting in ring expansion
Ring expansion and ring contraction reactions in the course of organic synthesis refer to a set of reactions which can lead to the expansion or contraction of an existing ring. This often makes it possible to access structures that would be dif ...
of the adjacent ring, with deprotionation unmasking the ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
functional group to complete the first line of the mechanism. This first product, a spirobicyclic ketone, is a spiro compound in its own right, and yields the further spiro carbinol and the alicyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.
The ...
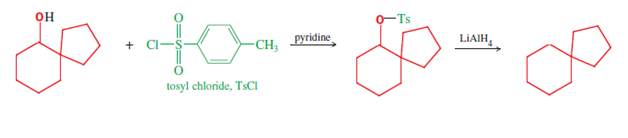
spiro hydrocarbon after two further reduction reactions. First, reduction of the carbonyl that ends the mechanism's first line provides the spiro carbinol starting material of the second line, which is needed for reduction to the alkane (shown). This latter reduction is accomplished using lithium aluminum hydride (LiAlH4), via the alcohol tosylate (formed using tosyl chloride). Hence this three reaction sequence provides three spiro compounds (ketone, alcohol, and alkane), of possible research or practical use.


Uses
Spiro forms of lactones and oxazines are frequently used as leuco dyes, frequently displaying chromism—reversible structural change between forms giving rise to colorless and colored appearances, especially in solution.Spiroaromaticity
Spiroaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single spiroatom. Although this spiro center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds.Etymology
A spiro compound, or spirane, from the Latin ''spīra'', meaning a twist or coil, For a further but less stable source of the same text that provides access to the relevant material, sesame access date. The Greek transcription, σπεῖρα, reflects the use of this cognate as one ancient Greek term to refer to a coil or related fold, see is a chemical compound, typically an organic compound, that presents a twisted structure of two or more rings (a ring system), in which 2 or 3 rings are linked together by one common atom, Note, the article co-authors, the Working Party of the IUPAC (1992-1998), were P. M. Giles, Jr., E. W. Godly, K.-H. Hellwich, A. K. Ikizler, M. V. Kisakürek, A. D. McNaught, G. P. Moss, J. Nyitrai, W. H. Powell, O. Weissbach, and A. Yerin. ''Also available online at'' ''Also available in German, with et al. indicating the same working party, at'' examples of which are shown at right.
Further reading
* * For a further but less stable source of the same text that provides access to the relevant material, sesame access date. * Examples of spiro natural products and their synthesis: * * The IUPAC documents on naming of spiro compounds: The full author (Working Party) list and a link to a German translation are provided in a corresponding footnote. ''Also available online at'' , same access date.
References
External links
{{Authority control Spiro compounds, *