Silylidene on:
[Wikipedia]
[Google]
[Amazon]
Silylene is a
 The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
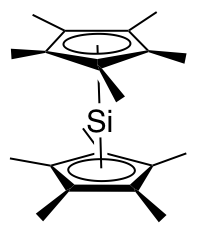 In one study diphenylsilylene is generated by
In one study diphenylsilylene is generated by  In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with
In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula SiH2. It is the silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
analog of methylene, the simplest carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet
A triplet is a set of three items, which may be in a specific order, or unordered. It may refer to:
Science
* A series of three nucleotide bases forming an element of the Genetic code
* J-coupling as part of Nuclear magnetic resonance spectrosc ...
state, silylene (and all of its derivatives) are singlets.
Silylenes are formal derivatives of silylene with its hydrogens replaced by other substituents. Most examples feature amido (NR2) or alkyl/aryl groups.
Silylenes have been proposed as reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s. They are carbene analog Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolate ...
s.
Synthesis and properties
Silylenes are generally synthesized bythermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
or photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of polysilanes, by silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
atom reactions (insertion
Insertion may refer to:
*Insertion (anatomy), the point of a tendon or ligament onto the skeleton or other part of the body
*Insertion (genetics), the addition of DNA into a genetic sequence
*Insertion, several meanings in medicine, see ICD-10-PCS
...
, addition
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol ) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication and Division (mathematics), division. ...
or abstraction), by pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
of silanes, or by reduction of 1,1-dihalosilane. It has long been assumed that the conversion of metallic Si to tetravalent silicon compounds proceeds via silylene intermediates:
:Si + Cl2 → SiCl2
:SiCl2 + Cl2 → SiCl4
Similar considerations apply to the direct process The direct process, also called the direct synthesis, Rochow process, and Müller-Rochow process is the most common technology for preparing organosilicon compounds on an industrial scale. It was first reported independently by Eugene G. Rochow and ...
, the reaction of methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
and bulk silicon.
Early observations of silylenes involved generation of dimethylsilylene by dechlorination of dimethyldichlorosilane
Dimethyldichlorosilane is a tetrahedral, organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made ...
:
:SiCl2(CH3)2 + 2 K → Si(CH3)2 + 2 KCl
The formation of dimethylsilylene was demonstrated by conducting the dechlorination in the presence of trimethylsilane, the trapped product being pentamethyldisilane:
:Si(CH3)2 + HSi(CH3)3 → (CH3)2Si(H)−Si(CH3)3
A room-temperature isolable ''N''-heterocyclic silylene is , first described in 1994 by Michael K. Denk Michael K. Denk (or Karl Michael Denk) is a Professor of chemistry at the University of Guelph, Ontario.Michael K. Denk'Personal Home Page at myprofile.cos.com. Accessed on 2009-12-07.
Karl Michael Denk (1992): ''Cyclische Metallamide : Synthese, ...
et al.
 The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
Related reactions
 In one study diphenylsilylene is generated by
In one study diphenylsilylene is generated by flash photolysis
Flash photolysis is a pump-probe laboratory technique, in which a sample is first excited by a strong pulse of light from a pulsed laser of nanosecond, picosecond, or femtosecond pulse width or by another short-pulse light source such as a fla ...
of a trisilane:
: In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with
In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with UV spectroscopy
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
at 520 nm and is short-lived with a chemical half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of two microseconds. Added methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
acts as a chemical trap In chemistry, a chemical trap is a chemical compound that is used to detect unstable compounds. The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trappin ...
with a second order rate constant of which is close to diffusion control.
See also
*Carbene analogs Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolate ...
* ''N''-heterocyclic silylene
* Silenes, R2Si=SiR2
* Silylium ions, protonated silylenes
References
{{Reflist Inorganic silicon compounds Free radicals