SSKI on:
[Wikipedia]
[Google]
[Amazon]
Potassium iodide is a
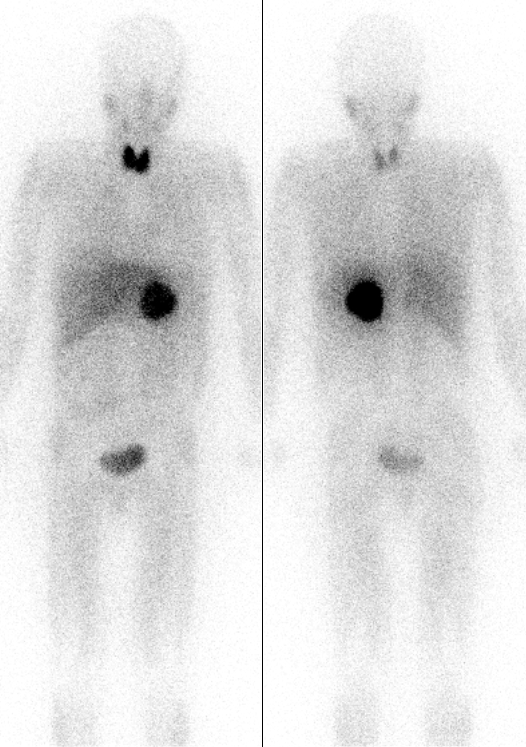 Thyroid iodine uptake blockade with potassium iodide is used in
Thyroid iodine uptake blockade with potassium iodide is used in
4 KI + 2 CO2 + O2 -> 2 K2CO3 + 2 I2
2 KI_ + Cl2_ -> 2 KCl_ + I2_
This reaction is employed in the isolation of iodine from natural sources. Air will oxidize iodide, as evidenced by the observation of a purple extract when aged samples of KI are rinsed with KI_ + I2_ -> KI3_
Unlike , salts can be highly water-soluble. Through this reaction, KI_ + 9AgNO3_ -> AgI_ + KNO3_
 In the Netherlands, the central storage of iodine-pills is located in
In the Netherlands, the central storage of iodine-pills is located in
World Health Organization's guidelines for iodine prophylaxis following a nuclear accident
{{portal bar, Medicine Potassium compounds Alkali metal iodides Disaster preparedness Expectorants Iodides Food additives Metal halides Photographic chemicals Radiobiology World Health Organization essential medicines Wikipedia medicine articles ready to translate Rock salt crystal structure Ophthalmology drugs
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
, medication
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field an ...
, and dietary supplement
A dietary supplement is a manufactured product intended to supplement one's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources or that are synthetic in orde ...
. It is a medication used for treating hyperthyroidism
Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidis ...
, in radiation emergencies
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, lar ...
, and for protecting the thyroid gland
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyr ...
when certain types of radiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is ...
s are used. In the third world
The term "Third World" arose during the Cold War to define countries that remained non-aligned with either NATO
The North Atlantic Treaty Organization (NATO, ; french: Organisation du traité de l'Atlantique nord, ), also called the Nor ...
it is also used for treating skin sporotrichosis
Sporotrichosis, also known as rose handler's disease, is a fungal infection that affects skin, lungs, bone and joint, and can be widespread. It presents with firm painless nodules that later ulcerate. It can be localized or widespread. The diseas ...
and phycomycosis
Phycomycosis is an uncommon condition of the gastrointestinal tract and skin most commonly found in dogs and horses. The condition is caused by a variety of molds and fungi, and individual forms include pythiosis, zygomycosis, and lagenidios ...
. It is a supplement used by people with low dietary intake of iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
. It is administered orally.
Common side effects include vomiting, diarrhea, abdominal pain, rash, and swelling of the salivary gland
The salivary glands in mammals are exocrine glands that produce saliva through a system of ducts. Humans have three paired major salivary glands ( parotid, submandibular, and sublingual), as well as hundreds of minor salivary glands. Salivar ...
s. Other side effects include allergic reactions
Allergies, also known as allergic diseases, refer a number of conditions caused by the hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic dermat ...
, headache
Headache is the symptom of pain in the face, head, or neck. It can occur as a migraine, tension-type headache, or cluster headache. There is an increased risk of depression in those with severe headaches.
Headaches can occur as a resul ...
, goitre
A goitre, or goiter, is a swelling in the neck resulting from an enlarged thyroid gland. A goitre can be associated with a thyroid that is not functioning properly.
Worldwide, over 90% of goitre cases are caused by iodine deficiency. The term is ...
, and depression. While use during pregnancy
Pregnancy is the time during which one or more offspring develops ( gestates) inside a woman's uterus (womb). A multiple pregnancy involves more than one offspring, such as with twins.
Pregnancy usually occurs by sexual intercourse, but ...
may harm the baby, its use is still recommended in radiation emergencies. Potassium iodide has the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
K I. Commercially it is made by mixing potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which expl ...
with iodine.
Potassium iodide has been used medically since at least 1820. It is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health s ...
. Potassium iodide is available as a generic medication
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active c ...
and over the counter
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescr ...
. Potassium iodide is also used for the iodization of salt
Iodised salt ( also spelled iodized salt) is table salt mixed with a minute amount of various salts of the element iodine. The ingestion of iodine prevents iodine deficiency. Worldwide, iodine deficiency affects about two billion people and is t ...
.
Medical uses
Dietary supplement
Potassium-iodide is a nutritional-supplement in animal feeds and also in the human diet. In humans it is the most common additive used for "iodizing"table salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
(a public health measure to prevent iodine deficiency
Iodine deficiency is a lack of the mineral (nutrient), trace element iodine, an essential nutrient in the diet (nutrition), diet. It may result in metabolic problems such as goiter, sometimes as an endemic goiter as well as congenital iodine defi ...
in populations that get little seafood). The oxidation of iodide causes slow loss of iodine content from iodised salt
Iodised salt (American and British English spelling differences#-ise, -ize (-isation, -ization), also spelled iodized salt) is table salt mixed with a minute amount of various salts of the element iodine. The ingestion of iodine prevents iodine ...
s that are exposed to excess air. The alkali metal iodide salt, over time and exposure to excess oxygen and carbon dioxide, slowly oxidizes to metal carbonate and elemental iodine, which then evaporates. Potassium iod''ate'' ( K I O3) is used to iodize some salts so that the iodine is not lost by oxidation. Dextrose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, usin ...
or sodium thiosulfate
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula . Typically it is available as the white or colorless pentahydrate, . The solid is an efflorescent (loses water readily) crystalline substance that dissolves well ...
are often added to iodized table salt to stabilize potassium iodide thus reducing loss of the volatile chemical.
Thyroid protection
 Thyroid iodine uptake blockade with potassium iodide is used in
Thyroid iodine uptake blockade with potassium iodide is used in nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emit ...
scintigraphy
Scintigraphy (from Latin ''scintilla'', "spark"), also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and ...
and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be used ...
(MIBG
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be used i ...
), which is used to image or treat neural tissue tumors, or iodinated fibrinogen
Fibrinogen (factor I) is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood ...
, which is used in fibrinogen scans to investigate clotting. These compounds contain iodine, but not in the iodide form. However, since they may be ultimately metabolized or break down to radioactive iodide, it is common to administer non-radioactive potassium iodide to ensure that iodide from these radiopharmaceuticals is not sequestered by the normal affinity of the thyroid for iodide.
U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
-approved dosing of potassium iodide for this purpose with iobenguane, is as follows (per 24 hours): infants less than 1 month old, 16 mg; children 1 month to 3 years, 32 mg; children 3 years to 18 years, 65 mg; adults 130 mg. However, some sources recommend alternative dosing regimens.
Not all sources are in agreement on the necessary ''duration'' of thyroid blockade, although agreement appears to have been reached about the ''necessity'' of blockade for both scintigraphic
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emittin ...
and therapeutic applications of iobenguane. Commercially available iobenguane is labeled with iodine-123
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2230 hours; the decay by electron capture to ...
, and product labeling recommends administration of potassium iodide 1 hour prior to administration of the radiopharmaceutical for all age groups, while the European Association of Nuclear Medicine recommends (for iobenguane labeled with either isotope), that potassium iodide administration begin one day prior to radiopharmaceutical administration, and continue until the day following the injection, with the exception of new-borns, who do not require potassium iodide doses following radiopharmaceutical injection.
Product labeling for diagnostic iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nu ...
iobenguane recommends potassium iodide administration one day before injection and continuing 5 to 7 days following administration, in keeping with the much longer half-life of this isotope and its greater danger to the thyroid. Iodine-131 iobenguane used for therapeutic purposes requires a different pre-medication duration, beginning 24–48 hours prior to iobenguane injection and continuing 10–15 days following injection.
Nuclear accidents
In 1982, the U.S.Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
approved potassium iodide to protect thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The t ...
glands from radioactive iodine
There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million year ...
involving accidents or fission emergencies. In an accidental event or attack on a nuclear power plant, or in nuclear bomb fallout, volatile fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
radionuclides
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
may be released. Of these products, (Iodine-131) is one of the most common and is particularly dangerous to the thyroid gland because it may lead to thyroid cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck. C ...
. By saturating the body with a source of stable iodide prior to exposure, inhaled or ingested tends to be excreted, which prevents radioiodine uptake by the thyroid. According to one 2000 study "KI administered up to 48 h before exposure can almost completely block thyroid uptake and therefore greatly reduce the thyroid absorbed dose. However, KI administration 96 h or more before exposure has no significant protective effect. In contrast, KI administration after exposure to radioiodine induces a smaller and rapidly decreasing blockade effect." For optimal prevention
Prevention may refer to:
Health and medicine
* Preventive healthcare, measures to prevent diseases or injuries rather than curing them or treating their symptoms
General safety
* Crime prevention, the attempt to reduce deter crime and crimi ...
, KI must be dosed daily until a risk of significant exposure to radioiodine by either inhalation or ingestion no longer exists.
Emergency 130 milligrams potassium iodide doses provide 100 mg iodide (the other 30 mg is the potassium in the compound), which is roughly 700 times larger than the normal nutritional need (see recommended dietary allowance
The Dietary Reference Intake (DRI) is a system of nutrition recommendations from the National Academy of Medicine (NAM) of the National Academies (United States). It was introduced in 1997 in order to broaden the existing guidelines known as R ...
) for iodine, which is 150 micrograms (0.15 mg) of iodine (as iodide) per day for an adult. A typical tablet weighs 160 mg, with 130 mg of potassium iodide and 30 mg of excipients
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts (thus often referred ...
, such as binding agent
A binder or binding agent is any material or substance that holds or draws other materials together to form a cohesive whole mechanically, chemically, by adhesion or cohesion.
In a more narrow sense, binders are liquid or dough-like substances th ...
s.
Potassium iodide cannot protect against any other mechanisms of radiation poisoning, nor can it provide any degree of protection against dirty bomb
A dirty bomb or radiological dispersal device is a radiological weapon that combines radioactive material with conventional explosives. The purpose of the weapon is to contaminate the area around the dispersal agent/conventional explosion with ...
s that produce radionuclides other than those of iodine.
The potassium iodide in iodized salt
Iodised salt ( also spelled iodized salt) is table salt mixed with a minute amount of various salts of the element iodine. The ingestion of iodine prevents iodine deficiency. Worldwide, iodine deficiency affects about two billion people and is t ...
is insufficient for this use. A likely lethal dose
In toxicology, the lethal dose (LD) is an indication of the lethal toxicity of a given substance or type of radiation. Because resistance varies from one individual to another, the "lethal dose" represents a dose (usually recorded as dose per kilog ...
of salt (more than a kilogramBy , the maximum allowable concentration of iodine in salt in the U.S. is .01%) would be needed to equal the potassium iodide in one tablet.
The World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level o ...
does not recommend KI prophylaxis for adults over 40 years, unless the radiation dose from inhaled radioiodine is expected to threaten thyroid function, because the KI side effects increase with age and may exceed the KI protective effects; "...unless doses to the thyroid from inhalation rise to levels threatening thyroid function, that is of the order of about 5 Gy. Such radiation doses will not occur far away from an accident site."
The U.S. Department of Health and Human Services
The United States Department of Health and Human Services (HHS) is a cabinet-level executive branch department of the U.S. federal government created to protect the health of all Americans and providing essential human services. Its motto is ...
restated these two years later as "The downward KI (potassium iodide) dose adjustment by age group, based on body size considerations, adheres to the principle of minimum effective dose. The recommended standard (daily) dose of KI for all school-age children is the same (65 mg). However, adolescents approaching adult size (i.e., >70 kg 54 lbs should receive the full adult dose (130 mg) for maximal block of thyroid radioiodine uptake. Neonates ideally should receive the lowest dose (16 mg) of KI."
SSKI (i.e., the "saturated solution of KI" rather than tablets) may be used in radioiodine-contamination emergencies (i.e., nuclear accidents) to "block" the thyroid's uptake of radioiodine, at a dose of two drops of SSKI per day for an adult. This is not the same as blocking the thyroid's release of thyroid hormone, for which the adult dose is different (and is actually higher by a factor of 7 or 8), and for which KI anti-radiation pills (not a common medical treatment form of KI) are not usually available in pharmacies, or normally used in hospitals, or by physicians. Although the two forms of potassium iodide are completely interchangeable, normally in practice the SSKI solution, which is the historical medical form of high dose iodine, is generally used for all medical purposes save for radioiodine prophylaxis. For protection of the thyroid against radioiodine (iodine-131) contamination, the convenient standard 130 mg KI pill is used, if available. As noted, the equivalent two drops of SSKI (equaling the dose of one KI pill) may be used for this purpose, if the pills are not available.
Side effects
There is reason for caution with prescribing the ingestion of high doses of potassium iodide and iodate, because their unnecessary use can cause conditions such as the Jod-Basedow phenomena, trigger and/or worsenhyperthyroidism
Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidis ...
and hypothyroidism
Hypothyroidism (also called ''underactive thyroid'', ''low thyroid'' or ''hypothyreosis'') is a disorder of the endocrine system in which the thyroid gland does not produce enough thyroid hormone. It can cause a number of symptoms, such as ...
, and then cause temporary or even permanent thyroid conditions. It can also cause sialadenitis
Sialadenitis (sialoadenitis) is inflammation of salivary glands, usually the major ones, the most common being the parotid gland, followed by submandibular and sublingual glands. It should not be confused with sialadenosis (sialosis) which is a no ...
(an inflammation of the salivary gland), gastrointestinal disturbances, and rashes. Potassium iodide is also not recommended for people with dermatitis herpetiformis
Dermatitis herpetiformis (DH) is a chronic autoimmune blistering skin condition, characterised by intensely itchy blisters filled with a watery fluid. DH is a cutaneous manifestation of coeliac disease, although the exact causal mechanism is not k ...
and hypocomplementemic vasculitis – conditions that are linked to a risk of iodine sensitivity.
There have been some reports of potassium iodide treatment causing swelling of the parotid gland
The parotid gland is a major salivary gland in many animals. In humans, the two parotid glands are present on either side of the mouth and in front of both ears. They are the largest of the salivary glands. Each parotid is wrapped around the m ...
(one of the three glands
In animals, a gland is a group of cells in an animal's body that synthesizes substances (such as hormones) for release into the bloodstream (endocrine gland) or into cavities inside the body or its outer surface (exocrine gland).
Structure
De ...
that secrete saliva), due to its stimulatory effects on saliva production.McCance; Huether. "Pathophysiology: The biological basis for disease in Adults and Children". 5th Edition. Elsievier Publishing
A saturated solution of KI (SSKI) is typically given orally in adult doses several times a day (5 drops of SSKI assumed to be mL) for thyroid blockade (to prevent the thyroid from excreting thyroid hormone) and occasionally this dose is also used, when iodide is used as an expectorant (the total dose is about one gram KI per day for an adult). The anti-radioiodine doses used for uptake blockade are lower, and range downward from 100 mg a day for an adult, to less than this for children (see table). All of these doses should be compared with the far lower dose of iodine needed in normal nutrition, which is only 150 μg per day (150 micrograms, not milligrams).
At maximal doses, and sometimes at much lower doses, side effects of iodide used for medical reasons, in doses of 1000 times the normal nutritional need, may include: acne, loss of appetite, or upset stomach (especially during the first several days, as the body adjusts to the medication). More severe side effects that require notification of a physician are: fever, weakness, unusual tiredness, swelling in the neck or throat, mouth sores, skin rash, nausea, vomiting, stomach pains, irregular heartbeat, numbness or tingling of the hands or feet, or a metallic taste in the mouth.
In the event of a radioiodine release the ingestion of prophylaxis potassium iodide, if available, or even iodate, would rightly take precedence over perchlorate administration, and would be the first line of defence in protecting the population from a radioiodine release. However, in the event of a radioiodine release too massive and widespread to be controlled by the limited stock of iodide and iodate prophylaxis drugs, then the addition of perchlorate ions to the water supply, or distribution of perchlorate tablets would serve as a cheap, efficacious, second line of defense against carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
radioiodine bioaccumulation.
The ingestion of goitrogen drugs is, much like potassium iodide also not without its dangers, such as hypothyroidism
Hypothyroidism (also called ''underactive thyroid'', ''low thyroid'' or ''hypothyreosis'') is a disorder of the endocrine system in which the thyroid gland does not produce enough thyroid hormone. It can cause a number of symptoms, such as ...
. In all these cases however, despite the risks, the prophylaxis benefits of intervention with iodide, iodate or perchlorate outweigh the serious cancer risk from radioiodine bioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance at a rate faster than that at which the substance is lost or eliminated ...
in regions where radioiodine has sufficiently contaminated the environment.
Potassium iodide in its raw form is a mild irritant and should be handled with gloves. Chronic overexposure can have adverse effects on the thyroid. Potassium iodide is a possible teratogen.
Industrial uses
KI is used with silver nitrate to makesilver iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is hig ...
(AgI), an important chemical in film photography. KI is a component in some disinfectants and hair treatment chemicals. KI is also used as a fluorescence quenching agent in biomedical research, an application that takes advantage of collisional quenching of fluorescent substances by the iodide ion. However, for several fluorophores addition of KI in μM-mM concentrations results in increase of fluorescence intensity, and iodide acts as fluorescence enhancer.
Potassium iodide is a component in the electrolyte of dye sensitised solar cells (DSSC) along with iodine.
Potassium iodide finds its most important applications in organic synthesis mainly in the preparation of aryl iodides in the Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts.
It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides ...
, starting from aryl amines. Aryl iodides are in turn used to attach aryl groups to other organics by nucleophilic substitution, with iodide ion as the leaving group.
Chemistry
Potassium iodide is anionic compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ...
which is made of the following ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s: . It crystallises in the sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35 ...
structure. It is produced industrially by treating KOH with iodine.
It is a white salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quanti ...
, which is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It absorbs water less readily than sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I ...
, making it easier to work with.
Aged and impure samples are yellow because of the slow oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of the salt to potassium carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and gl ...
and elemental iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
.
:Inorganic chemistry
Since theiodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine def ...
ion is a mild reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth m ...
, is easily oxidised to iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
() by powerful oxidising agents such as chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
:
:dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible wit ...
. As formed under acidic conditions, hydriodic acid
Hydroiodic acid (or hydriodic acid) is an aqueous solution of hydrogen iodide (HI). It is a strong acid, one that is ionized completely in an aqueous solution. It is colorless. Concentrated solutions are usually 48% to 57% HI.
Reactions
Hydr ...
(HI) is a stronger reducing agent.
Like other iodide salts, forms triiodide
In chemistry, triiodide usually refers to the triiodide ion, . This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been ...
() when combined with elemental iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
.
:iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
is used in redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant ...
s. Aqueous , "Lugol's solution
Lugol's iodine, also known as aqueous iodine and strong iodine solution, is a solution of potassium iodide with iodine in water. It is a medication and disinfectant used for a number of purposes. Taken by mouth it is used to treat thyrotoxicosi ...
", is used as a disinfectant and as an etchant for gold surfaces.
Potassium iodide and silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic ...
are used to make silver(I) iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is hig ...
, which is used for high speed photographic film and for cloud seeding
Cloud seeding is a type of weather modification that aims to change the amount or type of precipitation that falls from clouds by dispersing substances into the air that serve as cloud condensation or ice nuclei, which alter the microphysical ...
:
:Organic chemistry
KI serves as a source of iodide inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. A useful application is in the preparation of aryl iodides from arenediazonium salts
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
General properti ...
.
:
KI, acting as a source of iodide, may also act as a nucleophilic catalyst for the alkylation of alkyl chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
s, bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardan ...
s, or mesylate
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group ...
s.
History
Potassium iodide has been used medically since at least 1820. Some of the earliest uses included cures for syphilis,lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate ...
and mercury poisoning
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rash ...
.
Chernobyl
Potassium iodide's (KI) value as a radiation protective (thyroid blocking) agent was demonstrated following the Chernobyl nuclear reactor disaster in April, 1986. A saturated solution of potassium iodide (SSKI) was administered to 10.5 million children and 7 million adults inPoland
Poland, officially the Republic of Poland, , is a country in Central Europe. Poland is divided into Voivodeships of Poland, sixteen voivodeships and is the fifth most populous member state of the European Union (EU), with over 38 mill ...
as a preventative measure against accumulation of radioactive in the thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The t ...
gland.
Reports differ concerning whether people in the areas immediately surrounding Chernobyl itself were given the supplement. However the US Nuclear Regulatory Commission (NRC) reported, "thousands of measurements of I-131 (radioactive iodine) activity...suggest that the observed levels were lower than would have been expected had this prophylactic measure not been taken. The use of KI...was credited with permissible iodine content in 97% of the evacuees tested."
With the passage of time, people living in irradiated areas where KI was not available have developed thyroid cancer at epidemic levels, which is why the US Food and Drug Administration (FDA) reported "The data clearly demonstrate the risks of thyroid radiation... KI can be used oprovide safe and effective protection against thyroid cancer caused by irradiation."
Chernobyl also demonstrated that the need to protect the thyroid from radiation was greater than expected. Within ten years of the accident, it became clear that thyroid damage caused by released radioactive iodine was virtually the only adverse health effect that could be measured. As reported by the NRC, studies after the accident showed that "As of 1996, except for thyroid cancer, there has been no confirmed increase in the rates of other cancers, including leukemia, among the... public, that have been attributed to releases from the accident."
But equally important to the question of KI is the fact that radioactivity releases are not "local" events. Researchers at the World Health Organization accurately located and counted the residents with cancer from Chernobyl and were startled to find that "the increase in incidence f thyroid cancer
F, or f, is the sixth letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''ef'' (pronounced ), and the plural is ''efs''.
Hist ...
has been documented up to 500 km from the accident site... significant doses from radioactive iodine can occur hundreds of kilometers from the site, beyond emergency planning zones." Consequently, far more people than anticipated were affected by the radiation, which caused the United Nations to report in 2002 that "The number of people with thyroid cancer... has exceeded expectations. Over 11,000 cases have already been reported."
Nagasaki
These findings were consistent with studies of the effects of previous radioactivity releases. In 1945, millions of Japanese were exposed to radiation from nuclear weapons, and the effects can still be measured. Today, nearly half (44.8%) the survivors of Nagasaki studied have identifiable thyroid disease, with an editorial in ''The Journal of the American Medical Association'' reporting "it is remarkable that a biological effect from a single brief environmental exposure nearly 60 years in the past is still present and can be detected."Nuclear weapons testing
The development of thyroid cancer among residents in theNorth Pacific
The Pacific Ocean is the largest and deepest of Earth's five oceanic divisions. It extends from the Arctic Ocean in the north to the Southern Ocean (or, depending on definition, to Antarctica) in the south, and is bounded by the continen ...
from radioactive fallout following the United States' nuclear weapons testing in the 1950s (on islands nearly 200 miles downwind of the tests) were instrumental in the 1978 decision by the FDA
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food s ...
to issue a request for the availability of KI for thyroid protection in the event of a release from a commercial nuclear power plant or weapons-related nuclear incident. Noting that KI's effectiveness was "virtually complete" and finding that iodine in the form of KI was substantially superior to other forms including iodate (KIO3) in terms of safety, effectiveness, lack of side effects, and speed of onset, the FDA invited manufacturers to submit applications to produce and market KI.
Fukushima
It was reported on March 16, 2011, that potassium iodide tablets were given preventively to U.S. Naval air crew members flying within 70 nautical miles of theFukushima Daiichi Nuclear Power Plant
The is a disabled nuclear power plant located on a site in the towns of Ōkuma, Fukushima, Ōkuma and Futaba, Fukushima, Futaba in Fukushima Prefecture, Japan. The plant Fukushima Daiichi nuclear disaster, suffered major damage from the 2011 ...
damaged in the earthquake (8.9/9.0 magnitude) and ensuing tsunami on March 11, 2011. The measures were seen as precautions, and the Pentagon said no U.S. forces have shown signs of radiation poisoning. By March 20, the US Navy instructed personnel coming within 100 miles of the reactor to take the pills.
The Netherlands
 In the Netherlands, the central storage of iodine-pills is located in
In the Netherlands, the central storage of iodine-pills is located in Zoetermeer
Zoetermeer () is a city in the Western Netherlands, in the province of South Holland. The municipality covers an area of of which is water. A small village until the late 1960s, it had 6,392 inhabitants in 1950. By 2013 this had grown to 123,328 ...
, near The Hague
The Hague ( ; nl, Den Haag or ) is a list of cities in the Netherlands by province, city and municipalities of the Netherlands, municipality of the Netherlands, situated on the west coast facing the North Sea. The Hague is the country's ad ...
. In 2017, the Dutch government distributed pills to hundreds of thousands of residents who lived within a certain distance of nuclear power plants and met some other criteria.
Belgium
By 2020, potassium iodide tablets are made available free of charge for all residents in all pharmacies throughout the country.Formulations
Three companies (Anbex, Inc., Fleming Co, andRecipharm
Recipharm AB is one of the five largest pharmaceutical contract development and manufacturing organizations in the world, with production facilities in Sweden, France, Germany, Italy, Spain, Portugal, India and the UK as well as development site ...
of Sweden) have met the strict FDA requirements for manufacturing and testing of KI, and they offer products (IOSAT, ThyroShield, and ThyroSafe, respectively) which are available for purchase. In 2012, Fleming Co. sold all its product rights and manufacturing facility to other companies and no longer exists. ThyroShield is currently not in production. The Swedish manufacturing facility for Thyrosafe, a half-strength potassium iodide tablet for thyroid protection from radiation, was mentioned on the secret US 2008 Critical Foreign Dependencies Initiative leaked by Wikileaks in 2010.
Tablets of potassium iodide are supplied for emergency purposes related to blockade of radioiodine uptake, a common form of radiation poisoning due to environmental contamination by the short-lived fission product . Potassium iodide may also be administered pharmaceutically for thyroid storm
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyr ...
.
For reasons noted above, therapeutic drops of SSKI, or 130 mg tablets of KI as used for nuclear fission accidents, are not used as nutritional supplements, since an SSKI drop or nuclear-emergency tablet provides 300 to 700 times more iodine than the daily adult nutritional requirement. Dedicated nutritional iodide tablets containing 0.15 mg (150 micrograms (μg)) of iodide, from KI or from various other sources (such as kelp extract) are marketed as supplements, but they are not to be confused with the much higher pharmaceutical dose preparations.
Potassium iodide can be conveniently prepared in a saturated solution, abbreviated SSKI. This method of delivering potassium iodide doesn't require a method to weigh out the potassium iodide, thus allowing it to be used in an emergency situation. KI crystals are simply added to water until no more KI will dissolve and instead sits at the bottom of the container. With pure water, the concentration of KI in the solution depends only on the temperature. Potassium iodide is highly soluble in water thus SSKI is a concentrated source of KI. At 20 degrees Celsius the solubility of KI is 140-148 grams per 100 grams of water. Because the volumes of KI and water are approximately additive, the resulting SSKI solution will contain about 1.00 gram (1000 mg) KI per milliliter (mL) of solution. This is 100% weight/volume (note units of mass concentration) of KI (one gram KI per mL solution), which is possible because SSKI is significantly more dense than pure water—about 1.67 g/mL. Because KI is about 76.4% iodide by weight, SSKI contains about 764 mg iodide per mL. This concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
of iodide allows the calculation of the iodide dose per drop, if one knows the number of drops per milliliter. For SSKI, a solution more viscous than water, there are assumed to be 15 drops per mL; the iodide dose is therefore approximately 51 mg per drop. It is conventionally rounded to 50 mg per drop.
The term SSKI is also used, especially by pharmacists, to refer to a U.S.P. pre-prepared solution formula, made by adding KI to water to prepare a solution containing 1000 mg KI per mL solution (100% wt/volume KI solution), to closely approximate the concentration of SSKI made by saturation. This is essentially interchangeable with SSKI made by saturation, and also contains about 50 mg iodide per drop.
*Saturated solutions of potassium iodide can be an emergency treatment for hyperthyroidism
Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidis ...
(so-called thyroid storm
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyr ...
), as high amounts of iodide temporarily suppress secretion of thyroxine
File:Thyroid_system.svg, upright=1.5, The thyroid system of the thyroid hormones T3 and T4
rect 376 268 820 433 Thyroid-stimulating hormone
rect 411 200 849 266 Thyrotropin-releasing hormone
rect 297 168 502 200 Hypothalamus
rect 66 216 38 ...
from the thyroid gland. The dose typically begins with a loading dose, then mL SSKI (5 drops or 250 mg iodine as iodide), three times per day.
*Iodide solutions made from a few drops of SSKI added to drinks have also been used as expectorant
Mucoactive agents are a class of chemical agents that aid in the clearance of mucus or sputum from the upper and lower airways, including the lungs, bronchi, and trachea. Mucoactive drugs include expectorants, mucolytics, mucoregulators, and mucok ...
s to increase the water content of respiratory secretions and encourage effective coughing.
*SSKI has been proposed as a topical
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of class ...
treatment for sporotrichosis
Sporotrichosis, also known as rose handler's disease, is a fungal infection that affects skin, lungs, bone and joint, and can be widespread. It presents with firm painless nodules that later ulcerate. It can be localized or widespread. The diseas ...
, but no trials have been conducted to determine the efficacy or side effects of such treatment.
*Potassium iodide has been used for symptomatic treatment of erythema nodosum
Erythema nodosum (EN) is an inflammatory condition characterized by inflammation of the fat cells under the skin, resulting in tender red nodules or lumps that are usually seen on both shins. It can be caused by a variety of conditions, and typi ...
patients for persistent lesions whose cause remains unknown. It has been used in cases of erythema nodosum associated with Crohn's disease
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea (which may be bloody if inflammation is severe), fever, abdominal distension, ...
.
*Due to its high potassium content, SSKI is extremely bitter, and if possible it is administered in a sugar cube or small ball of bread. It may also be mixed into much larger volumes of juices.
*Neither SSKI or KI tablets form nutritional supplements, since the nutritional requirement for iodine is only 150 micrograms (0.15 mg) of iodide per day. Thus, a drop of SSKI provides 50/0.15 = 333 times the daily iodine requirement, and a standard KI tablet provides twice this much.
See also
*Elephant toothpaste
Elephant's toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide () using potassium iodide (KI) or yeast and warm water as a catalyst. How rapidly the reaction proceeds will depend on the concentration of hydrog ...
References
External links
*World Health Organization's guidelines for iodine prophylaxis following a nuclear accident
{{portal bar, Medicine Potassium compounds Alkali metal iodides Disaster preparedness Expectorants Iodides Food additives Metal halides Photographic chemicals Radiobiology World Health Organization essential medicines Wikipedia medicine articles ready to translate Rock salt crystal structure Ophthalmology drugs