SGOT on:
[Wikipedia]
[Google]
[Amazon]
Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase (GOT, SGOT), is a
 As a prototypical transaminase, AST relies on PLP (Vitamin B6) as a cofactor to transfer the amino group from aspartate or glutamate to the corresponding
As a prototypical transaminase, AST relies on PLP (Vitamin B6) as a cofactor to transfer the amino group from aspartate or glutamate to the corresponding
 # Internal
# Internal
AST
- Lab Tests Online
{{DEFAULTSORT:Aspartate Transaminase Liver function tests EC 2.6.1 Glutamate (neurotransmitter)
pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent a ...
(PLP)-dependent transaminase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α- keto acid. They are important in the synthesis of amino acids, which form proteins.
Function and mechanism
An amino acid ...
enzyme () that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
, heart
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to t ...
, skeletal muscle, kidneys
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; bloo ...
, brain
A brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It is located in the head, usually close to the sensory organs for senses such as vision. It is the most complex organ in a ve ...
, red blood cells and gall bladder. Serum AST level, serum ALT (alanine transaminase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first characte ...
) level, and their ratio (AST/ALT ratio
The AST/ALT ratio or De Ritis ratio is the ratio between the concentrations of the enzymes aspartate transaminase (AST) and alanine transaminase, aka alanine aminotransferase (ALT) in the blood of a human or animal. It is measured with a blood tes ...
) are commonly measured clinically as biomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
s for liver health. The tests are part of blood panels.
The half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of total AST in the circulation approximates 17 hours and, on average, 87 hours for ''mitochondrial'' AST. Aminotransferase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
Function and mechanism
An amino acid co ...
is cleared by sinusoidal cells in the liver.
Function
Aspartate transaminase catalyzes the interconversion of aspartate and α-ketoglutarate tooxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
and glutamate.
L-Aspartate (Asp) + α-ketoglutarate ↔ oxaloacetate + L-glutamate (Glu)
 As a prototypical transaminase, AST relies on PLP (Vitamin B6) as a cofactor to transfer the amino group from aspartate or glutamate to the corresponding
As a prototypical transaminase, AST relies on PLP (Vitamin B6) as a cofactor to transfer the amino group from aspartate or glutamate to the corresponding ketoacid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's En ...
. In the process, the cofactor shuttles between PLP and the pyridoxamine phosphate
Pyridoxamine is one form of vitamin B6. Chemically it is based on a pyridine ring structure, with hydroxyl, methyl, aminomethyl, and hydroxymethyl substituents. It differs from pyridoxine by the substituent at the 4-position. The hydroxyl at po ...
(PMP) form. The amino group transfer catalyzed by this enzyme is crucial in both amino acid degradation and biosynthesis. In amino acid degradation, following the conversion of α-ketoglutarate to glutamate, glutamate subsequently undergoes oxidative deamination to form ammonium ions, which are excreted as urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
. In the reverse reaction, aspartate may be synthesized from oxaloacetate, which is a key intermediate in the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
.
Isoenzymes
Two isoenzymes are present in a wide variety of eukaryotes. In humans: *GOT1
Aspartate aminotransferase, cytoplasmic is an enzyme that in humans is encoded by the ''GOT1'' gene.
Glutamic-oxaloacetic transaminase is a pyridoxal phosphate-dependent enzyme which exists in cytoplasmic and mitochondrial forms, GOT1 and GOT2, r ...
/cAST, the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
ic isoenzyme derives mainly from red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s and heart
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to t ...
.
* GOT2
Aspartate aminotransferase, mitochondrial is an enzyme that in humans is encoded by the ''GOT2'' gene. Glutamic-oxaloacetic transaminase is a pyridoxal phosphate-dependent enzyme which exists in cytoplasmic and inner-membrane mitochondrial forms, ...
/mAST, the mitochondrial isoenzyme is present predominantly in liver.
These isoenzymes are thought to have evolved from a common ancestral AST via gene duplication, and they share a sequence homology of approximately 45%.
AST has also been found in a number of microorganisms, including '' E. coli'', '' H. mediterranei'', and '' T. thermophilus''. In ''E. coli'', the enzyme is encoded by the ''aspC''gene and has also been shown to exhibit the activity of an aromatic-amino-acid transaminase ().
Structure

X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
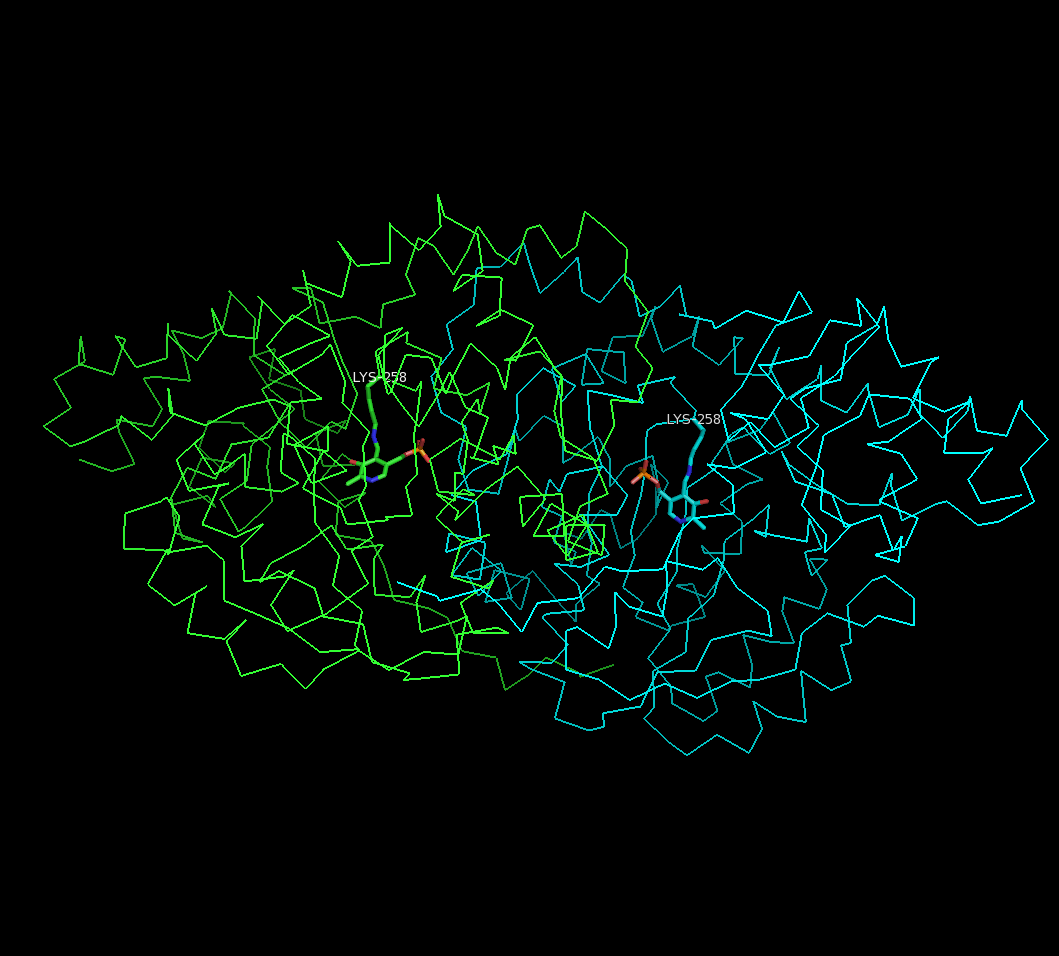
studies have been performed to determine the structure of aspartate transaminase from various sources, including chicken mitochondria, pig heart cytosol, and ''E. coli''. Overall, the three-dimensional polypeptide structure for all species is quite similar. AST is dimeric, consisting of two identical subunits, each with approximately 400 amino acid residues and a molecular weight of approximately 45 kD. Each subunit is composed of a large and a small domain, as well as a third domain consisting of the N-terminal residues 3-14; these few residues form a strand, which links and stabilizes the two subunits of the dimer. The large domain, which includes residues 48-325, binds the PLP cofactor via an aldimine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
linkage to the ε-amino group of Lys258. Other residues in this domain – Asp 222 and Tyr 225 – also interact with PLP via hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
. The small domain consists of residues 15-47 and 326-410 and represents a flexible region that shifts the enzyme from an "open" to a "closed" conformation upon substrate binding.
The two independent active sites are positioned near the interface between the two domains. Within each active site, a couple arginine residues are responsible for the enzyme's specificity for dicarboxylic acid substrates: Arg386 interacts with the substrate's proximal (α-)carboxylate group, while Arg292 complexes with the distal (side-chain) carboxylate.
In terms of secondary structure, AST contains both α and β elements. Each domain has a central sheet of β-strands with α-helices packed on either side.
Mechanism
Aspartate transaminase, as with all transaminases, operates via dual substrate recognition; that is, it is able to recognize and selectively bind two amino acids (Asp and Glu) with different side-chains. In either case, the transaminase reaction consists of two similar half-reactions that constitute what is referred to as a ping-pong mechanism. In the first half-reaction, amino acid 1 (e.g., L-Asp) reacts with the enzyme-PLP complex to generate ketoacid 1 (oxaloacetate) and the modified enzyme-PMP. In the second half-reaction, ketoacid 2 (α-ketoglutarate) reacts with enzyme-PMP to produce amino acid 2 (L-Glu), regenerating the original enzyme-PLP in the process. Formation of a racemic product (D-Glu) is very rare. The specific steps for the half-reaction of Enzyme-PLP + aspartate ⇌ Enzyme-PMP + oxaloacetate are as follows (see figure); the other half-reaction (not shown) proceeds in the reverse manner, with α-ketoglutarate as the substrate. # Internal
# Internal aldimine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
formation: First, the ε-amino group of Lys258 forms a Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
linkage with the aldehyde carbon to generate an internal aldimine.
# Transaldimination: The internal aldimine then becomes an external aldimine when the ε-amino group of Lys258 is displaced by the amino group of aspartate. This transaldimination reaction occurs via a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
by the deprotonated amino group of Asp and proceeds through a tetrahedral intermediate. As this point, the carboxylate groups of Asp are stabilized by the guanidinium
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experie ...
groups of the enzyme's Arg386 and Arg 292 residues.
# Quinonoid formation: The hydrogen attached to the a-carbon of Asp is then abstracted (Lys258 is thought to be the proton acceptor) to form a quinonoid intermediate.
# Ketimine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon– nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional si ...
formation: The quinonoid is reprotonated, but now at the aldehyde carbon, to form the ketimine intermediate.
# Ketimine hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
: Finally, the ketimine is hydrolyzed to form PMP and oxaloacetate.
This mechanism is thought to have multiple partially rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
s. However, it has been shown that the substrate binding step (transaldimination) drives the catalytic reaction forward.
Clinical significance
AST is similar toalanine transaminase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first characte ...
(ALT) in that both enzymes are associated with liver parenchymal
Parenchyma () is the bulk of functional substance in an animal organ or structure such as a tumour. In zoology it is the name for the tissue that fills the interior of flatworms.
Etymology
The term ''parenchyma'' is New Latin from the word ...
cells. The difference is that ALT is found predominantly in the liver, with clinically negligible quantities found in the kidneys, heart, and skeletal muscle, while AST is found in the liver, heart (cardiac muscle
Cardiac muscle (also called heart muscle, myocardium, cardiomyocytes and cardiac myocytes) is one of three types of vertebrate muscle tissues, with the other two being skeletal muscle and smooth muscle. It is an involuntary, striated muscle th ...
), skeletal muscle, kidneys, brain, and red blood cells. As a result, ALT is a more specific indicator of liver inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
than AST, as AST may be elevated also in diseases affecting other organs, such as myocardial infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may ...
, acute pancreatitis
Acute pancreatitis (AP) is a sudden inflammation of the pancreas. Causes in order of frequency include: 1) a gallstone impacted in the common bile duct beyond the point where the pancreatic duct joins it; 2) heavy alcohol use; 3) systemic disease; ...
, acute hemolytic anemia
Hemolytic anemia or haemolytic anaemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells (RBCs), either in the blood vessels (intravascular hemolysis) or elsewhere in the human body (extravascular). This most commonly ...
, severe burns, acute renal disease, musculoskeletal diseases, and trauma.
AST was defined as a biochemical marker for the diagnosis of acute myocardial infarction in 1954. However, the use of AST for such a diagnosis is now redundant and has been superseded by the cardiac troponins.
Laboratory tests should always be interpreted using the reference range from the laboratory that performed the test. Example reference ranges are shown below:
See also
*Alanine transaminase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first character ...
(ALT/ALAT/SGPT)
* Transaminases
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
Function and mechanism
An amino acid co ...
References
Further reading
* * * *External links
*AST
- Lab Tests Online
{{DEFAULTSORT:Aspartate Transaminase Liver function tests EC 2.6.1 Glutamate (neurotransmitter)