|
Aminotransferase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins. Function and mechanism An amino acid contains an amine (NH2) group. A keto acid contains a keto (=O) group. In transamination, the NH2 group on one molecule is exchanged with the =O group on the other molecule. The amino acid becomes a keto acid, and the keto acid becomes an amino acid. Most transaminases are protein enzymes. However, some transamination activities of the ribosome have been found to be catalyzed by ribozymes (RNA enzymes). Examples being the hammerhead ribozyme, the VS ribozyme and the hairpin ribozyme. Transaminases require the coenzyme pyridoxal phosphate, which is converted into pyridoxamine in the first half-reaction, when an amino acid is converted into a keto acid. Enzyme-bound pyridoxamine in turn reacts with pyruvate, oxaloacetate, or alpha-ketogluta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine Transaminase
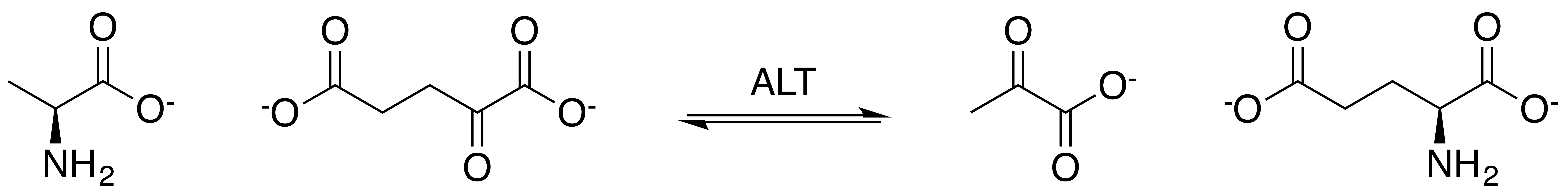
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first characterized in the mid-1950s by Arthur Karmen and colleagues. ALT is found in plasma and in various body tissues but is most common in the liver. It catalyzes the two parts of the alanine cycle. Serum ALT level, serum AST (aspartate transaminase) level, and their ratio (AST/ALT ratio) are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels. The half-life of ALT in the circulation approximates 47 hours. Aminotransferase is cleared by sinusoidal cells in the liver. Function ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate, the products of this reversible transamination reaction being pyruvate and L-glutamate. : L-alanine + α-ketoglutarate ⇌ pyruvate + L-glutama ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate Transaminase
Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase (GOT, SGOT), is a pyridoxal phosphate (PLP)-dependent transaminase enzyme () that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT (alanine transaminase) level, and their ratio (AST/ALT ratio) are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels. The half-life of total AST in the circulation approximates 17 hours and, on average, 87 hours for ''mitochondrial'' AST. Aminotransferase is cleared by Liver sinusoidal endothelial cell, sinusoidal cells in the liver. Function Aspartate transaminase catalyzes the inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism). Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. α-ketoglutarate acts as the predominant amino-group acceptor and produces glutamate as the new amino acid. :Aminoacid + α-ketoglutarate ↔ α-keto acid + glutamate Glutamate's amino group, in turn, is transferred to oxaloacetate in a second transamination reaction yielding aspartate. :Glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate Mechanism of action Transamination catalyzed by aminotransferase occurs in two stages. In the first step, the α amino group of an amino acid is transferred to the enzyme, producing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. .html" ;"title="/sup>">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it is located in the quadrant (anatomy), right upper quadrant of the abdomen, below the thoracic diaphragm, diaphragm. Its other roles in metabolism include the regulation of Glycogen, glycogen storage, decomposition of red blood cells, and the production of hormones. The liver is an accessory digestive organ that produces bile, an alkaline fluid containing cholesterol and bile acids, which helps the fatty acid degradation, breakdown of fat. The gallbladder, a small pouch that sits just under the liver, stores bile produced by the liver which is later moved to the small intestine to complete digestion. The liver's highly specialized biological tissue, tissue, consisting mostly of hepatocytes, regulates a w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Sugar
Glycaemia, also known as blood sugar level, blood sugar concentration, or blood glucose level is the measure of glucose concentrated in the blood of humans or other animals. Approximately 4 grams of glucose, a simple sugar, is present in the blood of a 70 kg (154 lb) human at all times. The body tightly regulates blood glucose levels as a part of metabolic homeostasis. Glucose is stored in skeletal muscle and liver cells in the form of glycogen; in fasting individuals, blood glucose is maintained at a constant level at the expense of glycogen stores in the liver and skeletal muscle. In humans, a blood glucose level of 4 grams, or about a teaspoon, is critical for normal function in a number of tissues, and the human brain consumes approximately 60% of blood glucose in fasting, sedentary individuals. A persistent elevation in blood glucose leads to glucose toxicity, which contributes to cell dysfunction and the pathology grouped together as complications of diabetes. Gl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ninhydrin
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is an organic compound with the formula C6H4(CO)2C(OH)2. It is used to detect ammonia and amines. Upon reaction with these amines, ninhydrin gets converted into deep blue or purple derivatives, which are called Ruhemann's purple. Ninhydrin is most commonly used to detect fingerprints, as the terminal amines of lysine residues in peptides and proteins sloughed off in fingerprints react with ninhydrin. Ninhydrin is a white solid that is soluble in ethanol and acetone. Ninhydrin can be considered as the hydrate of indane-1,2,3-trione. History Ninhydrin was discovered in 1910 by the German-English chemist Siegfried Ruhemann (1859–1943). In the same year, Ruhemann observed ninhydrin's reaction with amino acids. In 1954, Swedish investigators Oden and von Hofsten proposed that ninhydrin could be used to develop latent fingerprints. Uses Ninhydrin can also be used to monitor deprotection in solid phase peptide synthesis (Kaiser test). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogenate
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in at least one of these qualities. Heterogeneous Mixtures, in chemistry, is where certain elements are unwillingly combined and, when given the option, will separate. Etymology and spelling The words ''homogeneous'' and ''heterogeneous'' come from Medieval Latin ''homogeneus'' and ''heterogeneus'', from Ancient Greek ὁμογενής (''homogenēs'') and ἑτερογενής (''heterogenēs''), from ὁμός (''homos'', “same”) and ἕτερος (''heteros'', “other, another, different”) respectively, followed by γένος (''genos'', “kind”); ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |