Riemschneider Thiocarbamate Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Riemschneider thiocarbamate synthesis converts alkyl or aryl 
 The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrot ...
s to thiocarbamate
In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix ''thio-'' suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: ''O''-thiocarbamates, (ester ...
s under acidic conditions, followed by hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
with ice water. The reaction was discovered by the German chemist in 1951 as a more efficient method to produce thiocarbamates. Some references spell the name ''Riemenschneider''.
500px, center
The Riemschneider reaction can also be used to create the corresponding ''N''-substituted thiocarbamate from an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
or alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
.

Mechanism
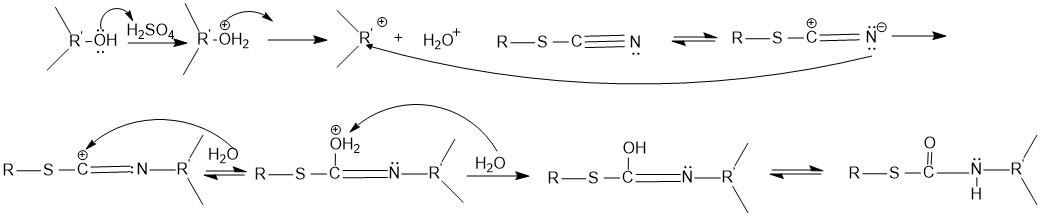
The mechanism for the conversion of an alcohol to the N-substituted thiocarbamate is shown below. The reaction proceeds under acidic conditions. The alcohol accepts a hydrogen ion from sulfuric acid to form a water, which then leaves, creating acarbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
. The mesomeric
Mesomeric Effect in Organic Chemistry
The Mesomeric Effect
The mesomeric effect (or resonance effect) in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the mole ...
form of the cyanogroup reacts with the carbocation. The carbocation is attacked by a water, which then loses an hydrogen to form the product. The product then undergoes hydrolysis to form the N-substituted thiocarbamate.
 The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
The reaction requires the formation of a carbocation and does not work for primary alcohols. Only secondary and tertiary alcohols undergo the Riemschneider reaction.
Uses and Limitations
The Riemschneider thiocarbamate synthesis for aromatic compounds does not work efficiently for ortho-substituted compounds such as ortho-carboxy, ortho-methoxy or ortho-nitro derivative compounds. The reaction is also not as efficient for compounds that are sensitive to concentrated acid, such as thiocyanophenols. The reaction works well for other compounds. Various thiocyanate compounds underwent the Riemschneider synthesis to form thiocarbamates, and all had melting points similar to the predicted value.References