Potential Energy Surface on:
[Wikipedia]
[Google]
[Amazon]
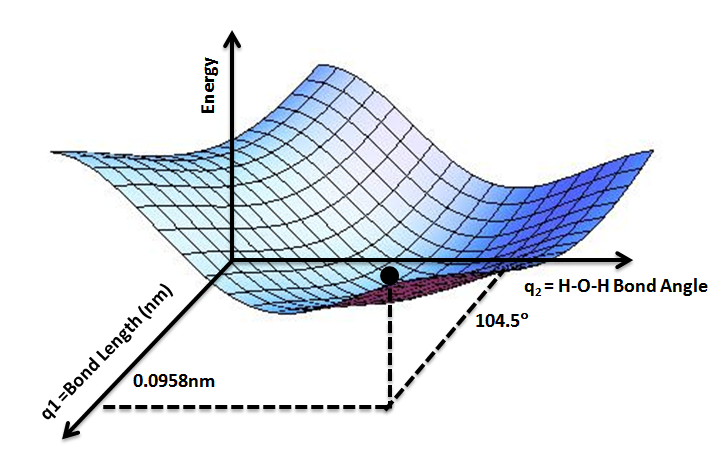 A potential energy surface (PES) or energy landscape describes the energy of a
A potential energy surface (PES) or energy landscape describes the energy of a
Errol G. Lewars, 2nd ed. (Springer 2011) p.21 The first semi-empirical calculation of a potential energy surface was proposed for the H + H2 reaction by Henry Eyring and
 A potential energy surface (PES) or energy landscape describes the energy of a
A potential energy surface (PES) or energy landscape describes the energy of a system
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its open system (systems theory), environment, is described by its boundaries, str ...
, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is ...
might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential
The Morse/Long-range potential (MLR potential) is an interatomic interaction model for the potential energy of a diatomic molecule. Due to the simplicity of the regular Morse potential (it only has three adjustable parameters), it is very limit ...
.
It is helpful to use the analogy of a landscape: for a system with two degrees of freedom
In many scientific fields, the degrees of freedom of a system is the number of parameters of the system that may vary independently. For example, a point in the plane has two degrees of freedom for translation: its two coordinates; a non-infinite ...
(e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground).
The PES concept finds application in fields such as physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
, chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
and biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
or computing the rates
Rate or rates may refer to:
Finance
* Rate (company), an American residential mortgage company formerly known as Guaranteed Rate
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate ...
of a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
. It can be used to describe all possible conformations of a molecular entity
In chemistry and physics, a molecular entity, or chemical entity, is "any constitutionally or isotopically distinct atom, molecule, ion, ion pair, radical, radical ion, complex, conformer, etc., identifiable as a separately distinguishable en ...
, or the spatial positions of interacting molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s in a system, or parameters and their corresponding energy levels, typically Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
. Geometrically, the energy landscape is the graph
Graph may refer to:
Mathematics
*Graph (discrete mathematics), a structure made of vertices and edges
**Graph theory, the study of such graphs and their properties
*Graph (topology), a topological space resembling a graph in the sense of discret ...
of the energy function across the configuration space of the system. The term is also used more generally in geometric perspectives to mathematical optimization
Mathematical optimization (alternatively spelled ''optimisation'') or mathematical programming is the selection of a best element, with regard to some criteria, from some set of available alternatives. It is generally divided into two subfiel ...
, when the domain of the loss function
In mathematical optimization and decision theory, a loss function or cost function (sometimes also called an error function) is a function that maps an event or values of one or more variables onto a real number intuitively representing some "cost ...
is the parameter space The parameter space is the space of all possible parameter values that define a particular mathematical model. It is also sometimes called weight space, and is often a subset of finite-dimensional Euclidean space.
In statistics, parameter spaces a ...
of some system.
Mathematical definition and computation
The geometry of a set of atoms can be described by a vector, , whose elements represent the atom positions. The vector could be the set of theCartesian coordinates
In geometry, a Cartesian coordinate system (, ) in a plane is a coordinate system that specifies each point uniquely by a pair of real numbers called ''coordinates'', which are the signed distances to the point from two fixed perpendicular o ...
of the atoms, or could also be a set of inter-atomic distances and angles.
Given , the energy as a function of the positions, , is the value of for all of interest. Using the landscape analogy from the introduction, ''E'' gives the height on the "energy landscape" so that the concept of a potential energy ''surface'' arises.
To study a chemical reaction using the PES as a function of atomic positions, it is necessary to calculate the energy for every atomic arrangement of interest. Methods of calculating the energy of a particular atomic arrangement of atoms are well described in the computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
article, and the emphasis here will be on finding approximations of to yield fine-grained energy-position information.
For very simple chemical systems or when simplifying approximations are made about inter-atomic interactions, it is sometimes possible to use an analytically derived expression for the energy as a function of the atomic positions. An example is the London
London is the Capital city, capital and List of urban areas in the United Kingdom, largest city of both England and the United Kingdom, with a population of in . London metropolitan area, Its wider metropolitan area is the largest in Wester ...
- Eyring- Polanyi-Sato potential for the system H + H2 as a function of the three H-H distances.
For more complicated systems, calculation of the energy of a particular arrangement of atoms is often too computationally expensive for large scale representations of the surface to be feasible. For these systems a possible approach is to calculate only a reduced set of points on the PES and then use a computationally cheaper interpolation method, for example Shepard interpolation, to fill in the gaps.
Application
A PES is a conceptual tool for aiding the analysis ofmolecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
and chemical reaction dynamics. Once the necessary points are evaluated on a PES, the points can be classified according to the first and second derivatives of the energy with respect to position, which respectively are the gradient
In vector calculus, the gradient of a scalar-valued differentiable function f of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p gives the direction and the rate of fastest increase. The g ...
and the curvature
In mathematics, curvature is any of several strongly related concepts in geometry that intuitively measure the amount by which a curve deviates from being a straight line or by which a surface deviates from being a plane. If a curve or su ...
. Stationary points (or points with a zero gradient) have physical meaning: energy minima correspond to physically stable chemical species and saddle point
In mathematics, a saddle point or minimax point is a Point (geometry), point on the surface (mathematics), surface of the graph of a function where the slopes (derivatives) in orthogonal directions are all zero (a Critical point (mathematics), ...
s correspond to transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s, the highest energy point on the reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate chosen to represent progress along a reaction pathway. Where possible it is usually a geometric parameter that changes during the conversion of one or more molecular e ...
(which is the lowest energy pathway connecting a chemical reactant to a chemical product).
The term is useful when examining protein folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, t ...
; while a protein can theoretically exist in a nearly infinite number of conformations along its energy landscape, in reality proteins fold (or "relax") into secondary and tertiary structures that possess the lowest possible free energy. The key concept in the energy landscape approach to protein folding is the ''folding funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. ...
'' hypothesis.
In catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, when designing new catalysts or refining existing ones, energy landscapes are considered to avoid low-energy or high-energy intermediates that could halt the reaction or demand excessive energy to reach the final products.
In glassing models, the local minima of an energy landscape correspond to metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
low temperature states
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
of a thermodynamic system
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics.
Thermodynamic systems can be passive and active according to internal processes. According to inter ...
.
In machine learning
Machine learning (ML) is a field of study in artificial intelligence concerned with the development and study of Computational statistics, statistical algorithms that can learn from data and generalise to unseen data, and thus perform Task ( ...
, artificial neural network
In machine learning, a neural network (also artificial neural network or neural net, abbreviated ANN or NN) is a computational model inspired by the structure and functions of biological neural networks.
A neural network consists of connected ...
s may be analyzed using analogous approaches. For example, a neural network may be able to perfectly fit the training set
In machine learning, a common task is the study and construction of algorithms that can learn from and make predictions on data. Such algorithms function by making data-driven predictions or decisions, through building a mathematical model from ...
, corresponding to a global minimum of zero loss, but overfitting
In mathematical modeling, overfitting is "the production of an analysis that corresponds too closely or exactly to a particular set of data, and may therefore fail to fit to additional data or predict future observations reliably". An overfi ...
the model
A model is an informative representation of an object, person, or system. The term originally denoted the plans of a building in late 16th-century English, and derived via French and Italian ultimately from Latin , .
Models can be divided in ...
("learning the noise" or "memorizing the training set"). Understanding when this happens can be studied using the geometry of the corresponding energy landscape.
Attractive and repulsive surfaces
Potential energy surfaces for chemical reactions can be classified as ''attractive'' or ''repulsive'' by comparing the extensions of the bond lengths in theactivated complex
In chemistry, an activated complex represents a collection of intermediate structures in a chemical reaction when bonds are breaking and forming. The activated complex is an arrangement of atoms in an arbitrary region near the saddle point
...
relative to those of the reactants and products. For a reaction of type A + B—C → A—B + C, the bond length extension for the newly formed A—B bond is defined as R*AB = RAB − R0AB, where RAB is the A—B bond length in the transition state and R0AB in the product molecule. Similarly for the bond which is broken in the reaction, R*BC = RBC − R0BC, where R0BC refers to the reactant molecule. Keith J. Laidler, ''Chemical Kinetics'' (3rd ed., Harper & Row 1987) p.461-8
For exothermic reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC define ...
s, a PES is classified as ''attractive'' (or ''early-downhill'') if R*AB > R*BC, so that the transition state is reached while the reactants are approaching each other. After the transition state, the A—B bond length continues to decrease, so that much of the liberated reaction energy is converted into vibrational energy of the A—B bond.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1998) p.272-4 An example is the harpoon reaction K + Br2 → K—Br + Br, in which the initial long-range attraction of the reactants leads to an activated complex resembling K+•••Br−•••Br. The vibrationally excited populations of product molecules can be detected by infrared chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction, i.e. a chemical reaction results in a flash or glow of light. A standard example of chemiluminescence in the laboratory se ...
.
In contrast the PES for the reaction H + Cl2 → HCl + Cl is ''repulsive'' (or ''late-downhill'') because R*HCl < R*ClCl and the transition state is reached when the products are separating. For this reaction in which the atom A (here H) is lighter than B and C, the reaction energy is released primarily as translational kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
of the products. For a reaction such as F + H2 → HF + H in which atom A is heavier than B and C, there is ''mixed'' energy release, both vibrational and translational, even though the PES is repulsive.
For endothermic reactions, the type of surface determines the type of energy which is most effective in bringing about reaction. Translational energy of the reactants is most effective at inducing reactions with an attractive surface, while vibrational excitation (to higher vibrational quantum number v) is more effective for reactions with a repulsive surface. As an example of the latter case, the reaction F + HCl(v=1) → Cl + HF is about five times faster than F + HCl(v=0) → Cl + HF for the same total energy of HCl.Atkins P. and de Paula J. ''Physical Chemistry'' (8th ed., W.H.Freeman 2006) p.889-890
History
The concept of a potential energy surface for chemical reactions was first suggested by the French physicist René Marcelin in 1913.Computational Chemistry: Introduction to the Theory and Applications of Molecular and Quantum MechanicsErrol G. Lewars, 2nd ed. (Springer 2011) p.21 The first semi-empirical calculation of a potential energy surface was proposed for the H + H2 reaction by Henry Eyring and
Michael Polanyi
Michael Polanyi ( ; ; 11 March 1891 – 22 February 1976) was a Hungarian-British polymath, who made important theoretical contributions to physical chemistry, economics, and philosophy. He argued that positivism is a false account of knowle ...
in 1931. Eyring used potential energy surfaces to calculate reaction rate constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants.
For a reaction between ...
s in the transition state theory in 1935.
H + H2 two-dimensional PES
Potential energy surfaces are commonly shown as three-dimensional graphs, but they can also be represented by two-dimensional graphs, in which the advancement of the reaction is plotted by the use of isoenergetic lines. The collinear system H + H2 is a simple reaction that allows a two-dimension PES to be plotted in an easy and understandable way. In this reaction, a hydrogen atom (H) reacts with a dihydrogen molecule (H2) by forming a new bond with one atom from the molecule, which in turn breaks the bond of the original molecule. This is symbolized as Ha + Hb–Hc → Ha–Hb + Hc. The progression of the reaction from reactants (H+H₂) to products (H-H-H), as well as the energy of the species that take part in the reaction, are well defined in the corresponding potential energy surface. Energy profiles describe potential energy as a function of geometrical variables (PES in any dimension are independent of time and temperature). We have different relevant elements in the 2-D PES: * The 2-D plot shows the minima points where we find reactants, the products and the saddle point or transition state. * The transition state is a maximum in the reaction coordinate and a minimum in the coordinate perpendicular to the reaction path. * The advance of time describes a trajectory in every reaction. Depending on the conditions of the reaction the process will show different ways to get to the product formation plotted between the 2 axes.See also
*Computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
* Energy minimization (or geometry optimization)
* Energy profile (chemistry)
*Potential well
A potential well is the region surrounding a local minimum of potential energy. Energy captured in a potential well is unable to convert to another type of energy ( kinetic energy in the case of a gravitational potential well) because it is cap ...
*Reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate chosen to represent progress along a reaction pathway. Where possible it is usually a geometric parameter that changes during the conversion of one or more molecular e ...
References
Bibliographie
{{Reaction mechanisms Quantum mechanics Potential theory Quantum chemistry