|
René Marcelin
René Marcelin (12 June 1885 – 24 September 1914) was a French physical chemist, who died in World War I at a young age. He was a pupil of Jean Baptiste Perrin at the Faculty of Sciences in Paris and performed theoretical studies in the field of chemical kinetics. Work René Marcelin developed the first theoretical treatment of the rate of chemical reactions that goes beyond a simple empirical description. He showed that the expression of the rate constant given by the Arrhenius equation had to be composed of two terms. In addition to the activation energy term, he considered that there had to be an activation entropy term. In 1910, Rene Marcelin introduced the concept of standard Gibbs energy of activation. In 1912, he treated the progress of a chemical reaction as a motion of a point in phase space In dynamical system theory, a phase space is a space in which all possible states of a system are represented, with each possible state corresponding to one uniq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gagny
Gagny () is a commune in the eastern suburbs of Paris, France. It is located from the center of Paris. Geography Location Gagny is located 10 km to the east of Paris. Until the law of 10 July 1964, the commune was part of the department of Seine-et-Oise. The redivision of the old departments of Seine and Seine-et-Oise then made this commune a part of Seine-Saint-Denis after an administrative transfer that went into effect 1 January 1968. History The priory was founded in the 11th century by Adela of Champagne. Gagny was the fiefdom of Étienne de Gagny, husband of Béatrice de Montfermeil in the 13th century. The priory lasted until 1771, the date de its suppression by the religious authority. Gagny had several castles, of which the most important, demolished in 1765, belonged to Dominique de Ferrari, Maître d'hôtel ordinaire of the king in 1660. In this park can be found the Saint-Fiacre spring, which supplied water to the park of Raincy at the end of 18th cent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Constant
In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction. For a reaction between reactants A and B to form product C the reaction rate is often found to have the form: r = k(T) mathrmm mathrm Here ''k''(''T'') is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reaction mechanism and can be determined experimentally. Elementary steps For an elementary step, there ''is'' a relationship between stoichiometry and rate law, as determined by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1885 Births
Events January–March * January 3– 4 – Sino-French War – Battle of Núi Bop: French troops under General Oscar de Négrier defeat a numerically superior Qing Chinese force, in northern Vietnam. * January 4 – The first successful appendectomy is performed by Dr. William W. Grant, on Mary Gartside. * January 17 – Mahdist War in Sudan – Battle of Abu Klea: British troops defeat Mahdist forces. * January 20 – American inventor LaMarcus Adna Thompson patents a roller coaster. * January 24 – Irish rebels damage Westminster Hall and the Tower of London with dynamite. * January 26 – Mahdist War in Sudan: Troops loyal to Mahdi Muhammad Ahmad conquer Khartoum; British commander Charles George Gordon is killed. * February 5 – King Leopold II of Belgium establishes the Congo Free State, as a personal possession. * February 9 – The first Japanese arrive in Hawaii. * February 16 – Charles Dow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Variational Transition-state Theory
Variational transition-state theory is a refinement of transition-state theory. When using transition-state theory to estimate a chemical reaction rate, the dividing surface is taken to be a surface that intersects a first-order saddle point and is also perpendicular to the reaction coordinate in all other dimensions. When using variational transition-state theory, the position of the dividing surface between Reagent, reactant and Product (chemistry), product regions is variationally optimized to minimize the reaction rate. This minimizes the effects of recrossing, and gives a much more accurate result. References {{reflist Chemical kinetics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
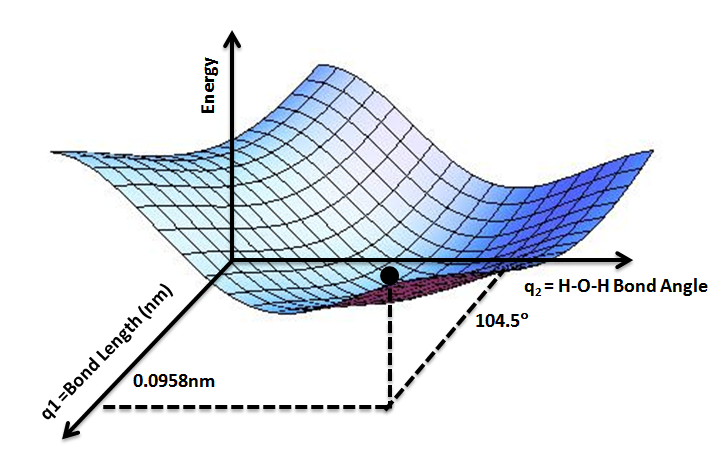
Potential Energy Surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential. It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground). The PES concept finds application in fields such as chemistry and physics, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
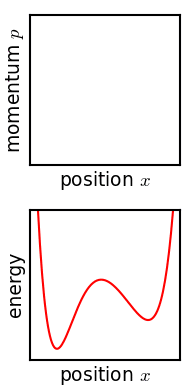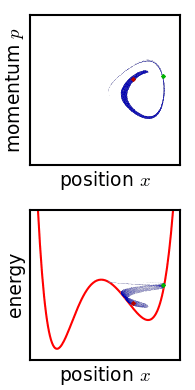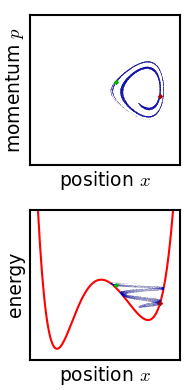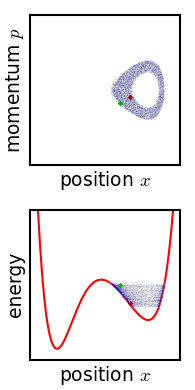
Phase Space
In dynamical system theory, a phase space is a space in which all possible states of a system are represented, with each possible state corresponding to one unique point in the phase space. For mechanical systems, the phase space usually consists of all possible values of position and momentum variables. It is the outer product of direct space and reciprocal space. The concept of phase space was developed in the late 19th century by Ludwig Boltzmann, Henri Poincaré, and Josiah Willard Gibbs. Introduction In a phase space, every degree of freedom or parameter of the system is represented as an axis of a multidimensional space; a one-dimensional system is called a phase line, while a two-dimensional system is called a phase plane. For every possible state of the system or allowed combination of values of the system's parameters, a point is included in the multidimensional space. The system's evolving state over time traces a path (a phase-space trajectory for the sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Of Activation
In chemical kinetics, the entropy of activation of a reaction is one of the two parameters (along with the enthalpy of activation) which are typically obtained from the temperature dependence of a reaction rate constant, when these data are analyzed using the Eyring equation of the transition state theory. The standard entropy of activation is symbolized and equals the change in entropy when the reactants change from their initial state to the activated complex or transition state ( = change, = entropy, = activation). It determines the preexponential factor of the Arrhenius equation for temperature dependence of reaction rates. The relationship depends on the molecularity of the reaction: for reactions in solution and unimolecular gas reactions , while for bimolecular gas reactions . In these equations is the base of natural logarithms, is the Planck constant, is the Boltzmann constant The Boltzmann constant ( or ) is the proportionality factor that relates the ave ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arrhenius Equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 1884 that the van 't Hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. This equation has a vast and important application in determining the rate of chemical reactions and for calculation of energy of activation. Arrhenius provided a physical justification and interpretation for the formula. Laidler, K. J. (1987) ''Chemical Kinetics'', Third Edition, Harper & Row, p. 42 Currently, it is best seen as an empirical relationship.Kenneth Connors, Chemical Kinetics, 1990, VCH Publishers It can be used to model the temperature variation of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally-induced processe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of overseas regions and territories in the Americas and the Atlantic, Pacific and Indian Oceans. Its metropolitan area extends from the Rhine to the Atlantic Ocean and from the Mediterranean Sea to the English Channel and the North Sea; overseas territories include French Guiana in South America, Saint Pierre and Miquelon in the North Atlantic, the French West Indies, and many islands in Oceania and the Indian Ocean. Due to its several coastal territories, France has the largest exclusive economic zone in the world. France borders Belgium, Luxembourg, Germany, Switzerland, Monaco, Italy, Andorra, and Spain in continental Europe, as well as the Netherlands, Suriname, and Brazil in the Americas via its overseas territories in French Guiana and Saint Martin. Its eighteen integral regions (five of which are overseas) span a combined area of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |