Pseudomonic Acid on:
[Wikipedia]
[Google]
[Amazon]
Mupirocin, sold under the brand name Bactroban among others, is a topical
 Mupirocin is used as a topical treatment for bacterial skin infections (for example,
Mupirocin is used as a topical treatment for bacterial skin infections (for example,

 Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture. Also present in mupirocin are pseudomonic acid B with an additional
Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture. Also present in mupirocin are pseudomonic acid B with an additional
antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
useful against superficial skin infections Skin and skin structure infections (SSSIs), also referred to as skin and soft tissue infections (SSTIs), or acute bacterial skin and skin structure infections (ABSSSIs), are infections of skin and associated soft tissues (such as loose connective ti ...
such as impetigo or folliculitis
Folliculitis is the infection and inflammation of one or more hair follicles. The condition may occur anywhere on hair-covered skin. The rash may appear as pimples that come to white tips on the face, chest, back, arms, legs, buttocks, or head.
A ...
. It may also be used to get rid of methicillin-resistant ''S. aureus'' (MRSA) when present in the nose without symptoms. Due to concerns of developing resistance
Resistance may refer to:
Arts, entertainment, and media Comics
* Either of two similarly named but otherwise unrelated comic book series, both published by Wildstorm:
** ''Resistance'' (comics), based on the video game of the same title
** ''T ...
, use for greater than ten days is not recommended. It is used as a cream or ointment applied to the skin.
Common side effects include itchiness and rash at the site of application, headache, and nausea. Long term use may result in increased growth of fungi. Use during pregnancy and breastfeeding appears to be safe. Mupirocin is in the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
class of medications. It works by blocking a bacteria's ability to make protein, which usually results in bacterial death.
Mupirocin was initially isolated in 1971 from ''Pseudomonas fluorescens
''Pseudomonas fluorescens'' is a common Gram-negative, rod-shaped bacterium. It belongs to the ''Pseudomonas'' genus; 16S rRNA analysis as well as phylogenomic analysis has placed ''P. fluorescens'' in the ''P. fluorescens'' group within the genu ...
''. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 203rd most commonly prescribed medication in the United States, with more than 2million prescriptions.
Medical uses
 Mupirocin is used as a topical treatment for bacterial skin infections (for example,
Mupirocin is used as a topical treatment for bacterial skin infections (for example, boil
A boil, also called a furuncle, is a deep folliculitis, which is an infection of the hair follicle. It is most commonly caused by infection by the bacterium ''Staphylococcus aureus'', resulting in a painful swollen area on the skin caused by an ...
s, impetigo, or open wounds), which are typically due to infection by ''Staphylococcus aureus'' or ''Streptococcus pyogenes''. It is also useful in the treatment of superficial methicillin-resistant ''Staphylococcus aureus'' (MRSA) infections. Mupirocin is inactive for most anaerobic bacteria, mycobacteria, mycoplasma, chlamydia, yeast, and fungi.
Intranasal mupirocin before surgery is effective for prevention of post-operative wound infection with ''Staphylcoccus aureus'' and preventative intranasal or catheter-site treatment is effective for reducing the risk of catheter site infection in persons treated with chronic peritoneal dialysis.
Resistance
Shortly after the clinical use of mupirocin began, strains of ''Staphylococcus aureus'' that were resistant to mupirocin emerged, with nares clearance rates of less than 30% success. Two distinct populations of mupirocin-resistant ''S. aureus'' were isolated. One strain possessed low-level resistance (MuL: MIC = 8–256 mg/L), and another possessed high-level resistance (MuH: MIC > 256 mg/L). Resistance in the MuL strains is probably due to mutations in the organism's wild-type isoleucyl-tRNA synthetase. In ''E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' IleRS, a single amino acid mutation was shown to alter mupirocin resistance. MuH is linked to the acquisition of a separate Ile synthetase gene, MupA. Mupirocin is not a viable antibiotic against MuH strains. Other antibiotic agents, such as azelaic acid
Azelaic acid (AzA) is an organic compound with the formula HOOC(CH2)7 COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers an ...
, nitrofurazone, silver sulfadiazine
Silver sulfadiazine, sold under the brand Silvadene among others, is a topical antibiotic used in partial thickness and full thickness burns to prevent infection. Tentative evidence has found other antibiotics to be more effective, and therefore ...
, and ramoplanin
Ramoplanin (INN) is a glycolipodepsipeptide antibiotic drug derived from strain ATCC 33076 of ''Actinoplanes''. It is effective against Gram-positive bacteria.
Mechanism
It exerts its bacteriocidal effect by inhibiting cell wall biosynthesi ...
have been shown to be effective against MuH strains.
Most strains of '' Cutibacterium acnes'', a causative agent in the skin disease acne vulgaris
Acne, also known as ''acne vulgaris'', is a long-term skin condition that occurs when dead skin cells and oil from the skin clog hair follicles. Typical features of the condition include blackheads or whiteheads, pimples, oily skin, and po ...
, are naturally resistant to mupirocin.
Most strains of ''Pseudomonas fluorescens'' are also resistant to mupirocin as they produce the antibiotic and it’s possible other species of '' Pseudomonas'' may be resistant as well.
The mechanism of action of mupirocin differs from other clinical antibiotics, rendering cross-resistance to other antibiotics unlikely. However, the MupA gene may co-transfer with other antibacterial resistance genes. This has been observed already with resistance genes for triclosan, tetracycline, and trimethoprim. It may also result in overgrowth of non-susceptible organisms.
Mechanism of action
Pseudomonic acid inhibits isoleucine tRNA synthetase in bacteria, leading to depletion of isoleucyl-tRNA and accumulation of the corresponding uncharged tRNA. Depletion of isoleucyl-tRNA results in inhibition of protein synthesis. The uncharged form of the tRNA binds to the aminoacyl-tRNA binding site of ribosomes, triggering the formation of (p)ppGpp, which in turn inhibits RNA synthesis. The combined inhibition of protein synthesis and RNA synthesis results in bacteriostasis. This mechanism of action is shared withfuranomycin
Furanomycin is an isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic aci ...
, an analog
Analog or analogue may refer to:
Computing and electronics
* Analog signal, in which information is encoded in a continuous variable
** Analog device, an apparatus that operates on analog signals
*** Analog electronics, circuits which use analo ...
of isoleucine.
Biosynthesis

 Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture. Also present in mupirocin are pseudomonic acid B with an additional
Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture. Also present in mupirocin are pseudomonic acid B with an additional hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
at C8, pseudomonic acid C with a double bond between C10 and C11, instead of the epoxide of PA-A, and pseudomonic acid D with a double bond at C4` and C5` in the 9-hydroxy-nonanoic acid portion of mupirocin.
Biosynthesis of pseudomonic acid A
The 74 kb mupirocin gene cluster contains six multi-domainenzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
and twenty-six other peptides (Table 1). Four large multi-domain type I polyketide synthase (PKS) proteins are encoded, as well as several single function enzymes with sequence similarity to type II PKSs. Therefore, it is believed that mupirocin is constructed by a mixed type I and type II PKS system. The mupirocin cluster exhibits an atypical acyltransferase
Acyltransferase is a type of transferase enzyme that acts upon acyl groups.
Examples include:
* Glyceronephosphate O-acyltransferase
* Lecithin-cholesterol acyltransferase
*Long-chain-alcohol O-fatty-acyltransferase
In enzymology, a long-chain- ...
(AT) organization, in that there are only two AT domains, and both are found on the same protein, MmpC. These AT domains are the only domains present on MmpC, while the other three type I PKS proteins contain no AT domains. The mupirocin pathway also contains several tandem acyl carrier protein doublets or triplets. This may be an adaptation to increase the throughput rate or to bind multiple substrates simultaneously.
Pseudomonic acid A is the product of an esterification between the 17C polyketide monic acid and the 9C fatty acid 9-hydroxy-nonanoic acid. The possibility that the entire molecule is assembled as a single polyketide with a Baeyer-Villiger oxidation inserting an oxygen into the carbon backbone has been ruled out because C1 of monic acid and C9' of 9-hydroxy-nonanoic acid are both derived from C1 of acetate.
Monic acid biosynthesis
Biosynthesis of the 17C monic acid unit begins on MmpD (Figure 1). One of the AT domains from MmpC may transfer an activated acetyl group from acetyl-Coenzyme A (CoA) to the first ACP domain. The chain is extended by malonyl-CoA, followed by a SAM-dependentmethylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
at C12 (see Figure 2 for PA-A numbering) and reduction of the B-keto group to an alcohol. The dehydration (DH) domain in module 1 is predicted to be non-functional due to a mutation in the conserved active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
region. Module 2 adds another two carbons by the malonyl-CoA extender unit, followed by ketoreduction (KR) and dehydration. Module three adds a malonyl-CoA extender unit, followed by SAM-dependent methylation at C8, ketoreduction, and dehydration. Module 4 extends the molecule with a malonyl-CoA unit followed by ketoreduction.
Assembly of monic acid is continued by the transfer of the 12C product of MmpD to MmpA. Two more rounds of extension with malonyl-CoA units are achieved by module 5 and 6. Module 5 also contains a KR domain.
Post-PKS tailoring
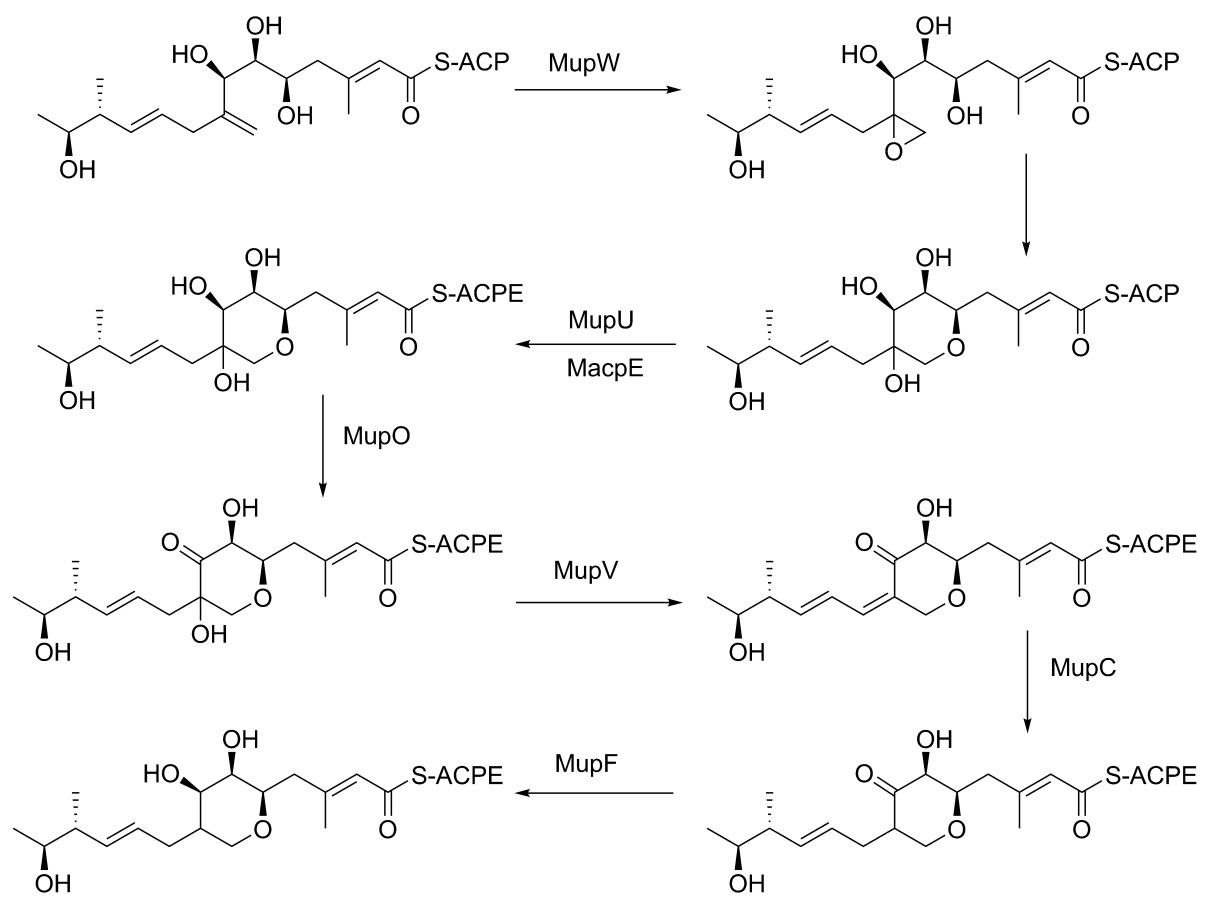
The keto group at C3 is replaced with a methyl group in a multi-step reaction (Figure 3). MupG begins by decarboxylating a malonyl-ACP. The alpha carbon of the resulting acetyl-ACP is linked to C3 of the polyketide chain by MupH. This intermediate is dehydrated and decarboxylated by MupJ and MupK, respectively. The formation of the pyran ring requires many enzyme-mediated steps (Figure 4). The double bond between C8 and C9 is proposed to migrate to between C8 and C16.Gene knockout
A gene knockout (abbreviation: KO) is a genetic technique in which one of an organism's genes is made inoperative ("knocked out" of the organism). However, KO can also refer to the gene that is knocked out or the organism that carries the gene kno ...
experiments of mupO, mupU, mupV, and macpE have eliminated PA-A production. PA-B production is not removed by these knockouts, demonstrating that PA-B is not created by hydroxylating PA-A. A knockout of mupW eliminated the pyran ring, identifying MupW as being involved in ring formation. It is not known whether this occurs before or after the esterification of monic acid to 9-hydroxy-nonanoic acid.
The epoxide of PA-A at C10-11 is believed to be inserted after pyran formation by a cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
such as MupO. A gene knockout of mupO abolished PA-A production but PA-B, which also contains the C10-C11 epoxide, remained. This indicates that MupO is either not involved or is not essential for this epoxidation step.
9-Hydroxy-nonanoic acid biosynthesis
The nine-carbon fatty acid 9-hydroxy-nonanoic acid (9-HN) is derived as a separate compound and later esterified to monic acid to form pseudomonic acid. 13C labeledacetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
feeding has shown that C1-C6 are constructed with acetate in the canonical fashion of fatty acid synthesis. C7' shows only C1 labeling of acetate, while C8' and C9' show a reversed pattern of 13C labeled acetate. It is speculated that C7-C9 arises from a 3-hydroxypropionate starter unit, which is extended three times with malonyl-CoA and fully reduced to yield 9-HN. It has also been suggested that 9-HN is initiated by 3-hydroxy-3-methylglutaric acid (HMG). This latter theory was not supported by feeding of -14Cor ,6-13C2HMG.
It is proposed that MmpB to catalyzes the synthesis of 9-HN (Figure 5). MmpB contains a KS, KR, DH, 3 ACPs, and a thioesterase (TE) domain. It does not contain an enoyl reductase (ER) domain, which would be required for the complete reduction to the nine-carbon fatty acid. MupE is a single-domain protein that shows sequence similarity to known ER domains and may complete the reaction. It also remains possible that 9-hydroxy-nonanoic acid is derived partially or entirely from outside of the mupirocin cluster.
References
External links
* {{Portal bar, Medicine Antibiotics Carboxylate esters Epoxides Fatty acids GSK plc brands World Health Organization essential medicines Wikipedia medicine articles ready to translate