Progestogen Esters on:
[Wikipedia]
[Google]
[Amazon]
 A progestogen ester is an
A progestogen ester is an

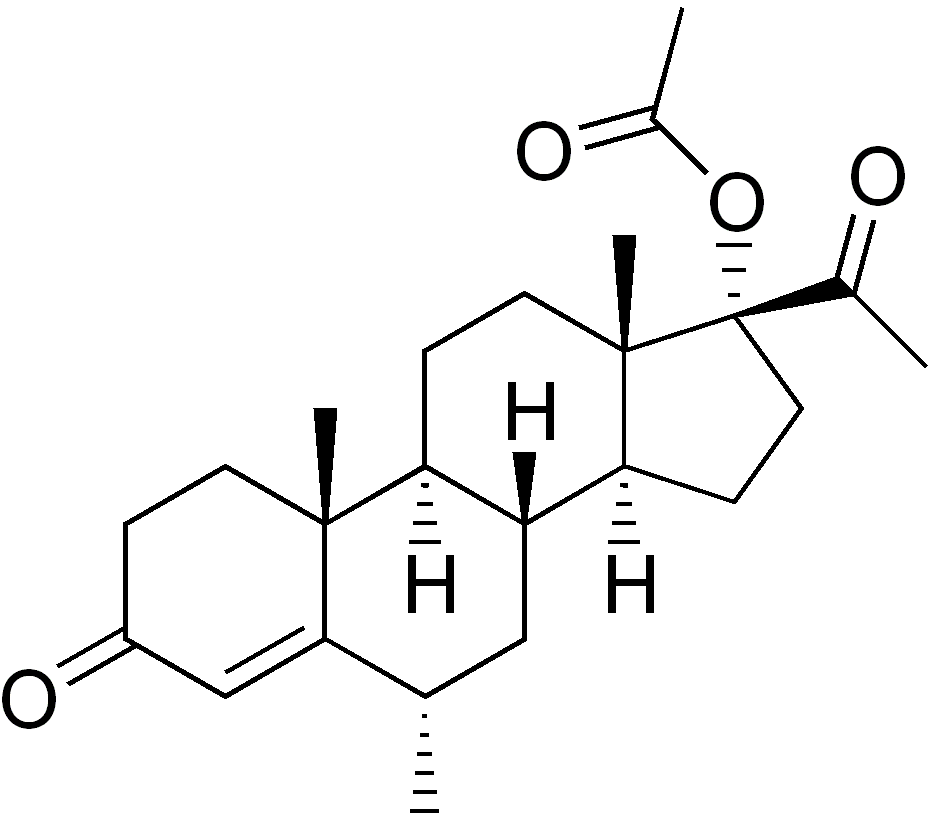 Medroxyprogesterone acetate (Provera) entered clinical use and became widely marketed, largely superseding the 17α-hydroxyprogesterone esters. A variety of analogues of medroxyprogesterone acetate, such as
Medroxyprogesterone acetate (Provera) entered clinical use and became widely marketed, largely superseding the 17α-hydroxyprogesterone esters. A variety of analogues of medroxyprogesterone acetate, such as

 Although it cannot be esterified, progesterone possesses ketone groups at the C3 and C20 positions, and for this reason, it is possible to
Although it cannot be esterified, progesterone possesses ketone groups at the C3 and C20 positions, and for this reason, it is possible to
 While not esters, C3 and C20
While not esters, C3 and C20
springer.com Some 19-nortestosterone progestins, including the marketed progestins norgestimate and norelgestromin and the non-marketed progestin norethisterone acetate oxime, are C3 oximes, although they have potent progestogenic activity of their own and are not necessarily prodrugs of the corresponding ketones.
ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
of a progestogen or progestin (a synthetic progestogen). The prototypical progestogen is progesterone, an endogenous sex hormone
Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effect ...
. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral
The word oral may refer to:
Relating to the mouth
* Relating to the mouth, the first portion of the alimentary canal that primarily receives food and liquid
**Oral administration of medicines
** Oral examination (also known as an oral exam or or ...
bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
, lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lip ...
, and elimination half-life
Biological half-life (also known as elimination half-life, pharmacologic half-life) is the time taken for concentration of a biological substance (such as a medication) to decrease from its maximum concentration ( Cmax) to half of Cmax in the bl ...
. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many (though not all) steroid esters function as prodrugs.
Esterification is particularly salient in the case of progesterone because progesterone itself shows very poor oral pharmacokinetics and is thus ineffective when taken orally. Unmodified, it has an elimination half-life of only 5 minutes, and is almost completely inactivated by the liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
during first-pass metabolism
The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism whereby the concentration of a drug, specifically when administered orally, is greatly reduced before it reaches the system ...
. Micronization
Micronization is the process of reducing the average diameter of a solid material's particles. Traditional techniques for micronization focus on mechanical means, such as milling and grinding. Modern techniques make use of the properties of supe ...
, however, has allowed for progesterone to be effective orally, although oral micronized progesterone was not developed until recent years.
Examples of important progestogen esters include the 17α-hydroxyprogesterone
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many o ...
derivative
In mathematics, the derivative of a function of a real variable measures the sensitivity to change of the function value (output value) with respect to a change in its argument (input value). Derivatives are a fundamental tool of calculus. ...
s medroxyprogesterone acetate, megestrol acetate
Megestrol acetate (MGA), sold under the brand name Megace among others, is a progestin medication which is used mainly as an appetite stimulant to treat wasting syndromes such as cachexia.https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/ ...
, cyproterone acetate
Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent condition ...
, and hydroxyprogesterone caproate
Hydroxyprogesterone caproate (OHPC), sold under the brand names Proluton and Makena among others, is a progestin medication which is used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological diso ...
, the 19-norprogesterone derivative nomegestrol acetate
Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders.http://www.ema. ...
, and the 19-nortestosterone derivatives norethisterone acetate
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gyn ...
and norethisterone enanthate
Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of hormonal birth control which is used to prevent pregnancy in women. It is used both as a form of progestogen-only injectable birth control and in combined inject ...
.
Progestogen esters
Estrogen
Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal ac ...
s were discovered in 1929, and beginning in 1936, a variety of estradiol ester
This is a list of estrogen esters, or ester prodrugs of estrogens. It includes esters, as well as ethers, of steroidal estrogens like estradiol, estrone, and estriol and of nonsteroidal estrogens like the stilbestrols diethylstilbestrol and hexes ...
s, such as estradiol benzoate
Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in t ...
and estradiol dipropionate
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the ...
, were introduced for clinical use. Testosterone ester
This is a list of androgen esters, including esters (as well as ethers) of natural androgens like testosterone and dihydrotestosterone (DHT) and synthetic anabolic–androgenic steroids (AAS) like nandrolone (19-nortestosterone).
Esters of nat ...
s, such as testosterone propionate
Testosterone propionate, sold under the brand name Testoviron among others, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels in men. It has also been used to treat breast cance ...
and testosterone phenylacetate
Testosterone phenylacetate (TPA; brand names Perandren, Androject) is an androgen and anabolic steroid and a testosterone ester. Analogously to estradiol benzoate having been one of the first estrogen esters to be introduced, testosterone phenyl ...
, were also introduced around this time. In contrast to estradiol
Estradiol (E2), also spelled oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is responsible for the development o ...
and testosterone
Testosterone is the primary sex hormone and anabolic steroid in males. In humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristi ...
, progesterone proved more difficult to esterify. In fact, esterification involves the replacement of a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
group with an alkoxy
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also ...
group, and unlike estradiol and testosterone, progesterone does not possess any hydroxyl groups, so it is actually not chemically possible to esterify progesterone itself. The first progestogen esters were not introduced until the mid-1950s, and were esters of 17α-hydroxyprogesterone
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many o ...
(which, unlike progesterone, has a hydroxyl group available for esterification) rather than of progesterone; they included 17α-hydroxyprogesterone caproate (Delalutin, Proluton) and 17α-hydroxyprogesterone acetate (Prodrox). The following quote of de Médicis Sajous et al. (1961) details the development of progestogen esters:
 Medroxyprogesterone acetate (Provera) entered clinical use and became widely marketed, largely superseding the 17α-hydroxyprogesterone esters. A variety of analogues of medroxyprogesterone acetate, such as
Medroxyprogesterone acetate (Provera) entered clinical use and became widely marketed, largely superseding the 17α-hydroxyprogesterone esters. A variety of analogues of medroxyprogesterone acetate, such as chlormadinone acetate
Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hor ...
, cyproterone acetate
Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent condition ...
, and megestrol acetate
Megestrol acetate (MGA), sold under the brand name Megace among others, is a progestin medication which is used mainly as an appetite stimulant to treat wasting syndromes such as cachexia.https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/ ...
, were subsequently developed and introduced as well. Progestogen esters of other groups of progestins have also been introduced, including the 19-norprogesterone derivatives gestonorone caproate
Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the en ...
, segesterone acetate (nestorone), nomegestrol acetate
Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders.http://www.ema. ...
, and norgestomet
Norgestomet, or norgestamet, sold under the brand name Syncro-Mate B and Crestar, is a progestin medication which is used in veterinary medicine to control estrus and ovulation in cattle.
Uses
Veterinary
Norgestomet is used in veterinary medi ...
(11β-methyl-17α-acetoxy-19-norprogesterone) and the 19-nortestosterone derivatives etynodiol diacetate
Etynodiol diacetate, or ethynodiol diacetate, sold under the brand names Demulen and Femulen among others, is a progestin medication which is used in birth control pills. The medication is available only in combination with an estrogen. It is t ...
, norethisterone acetate
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gyn ...
, norethisterone enanthate
Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of hormonal birth control which is used to prevent pregnancy in women. It is used both as a form of progestogen-only injectable birth control and in combined inject ...
, and quingestanol acetate
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.
Quingestanol acetate is a progestin, or a synt ...
.
Although esters of steroidal androgens and estrogens are generally inactive themselves and act as prodrugs, the same is not true for many progestogen esters. For instance, esters of 17α-hydroxyprogesterone derivatives, such as hydroxyprogesterone caproate, medroxyprogesterone acetate, and cyproterone acetate, are highly active themselves (in fact, they are far more active than their unesterified forms) and are not prodrugs, forming little or none of their parent compounds (in the cases of the examples given, hydroxyprogesterone, medroxyprogesterone
Medroxyprogesterone (MP), is a progestin which is not used medically. A derivative, medroxyprogesterone acetate (MPA), is used as a medication in humans, and is far more widely known in comparison. ''Medroxyprogesterone'' is sometimes used as a ...
, and cyproterone
Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, p ...
, respectively). On the other hand, esters of 19-nortestosterone derivatives, such as etynodiol diacetate, norethisterone acetate, norethisterone enanthate, and quingestanol acetate, are all prodrugs.
Progestogen ethers
ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
ify it; that is, progesterone ethers are possible. Quingestrone
Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken ...
(Enol-Luteovis) is a progesterone ether (specifically, the 3-cyclopentyl ether of progesterone) that has been marketed in Italy
Italy ( it, Italia ), officially the Italian Republic, ) or the Republic of Italy, is a country in Southern Europe. It is located in the middle of the Mediterranean Sea, and its territory largely coincides with the homonymous geographical ...
as an oral contraceptive Oral contraceptives, abbreviated OCPs, also known as birth control pills, are medications taken by mouth for the purpose of birth control.
Female
Two types of female oral contraceptive pill, taken once per day, are widely available:
* The combi ...
. Quingestrone is a variant of progesterone with improved pharmacokinetics, including higher potency, oral activity, greater lipophilicity, and a longer half-life. Two other progestogens, pentagestrone
Pentagestrone (), also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, pentagestrone acetate
Pentagestrone acetate (PGA), ...
(never marketed) and pentagestrone acetate
Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3- cyclopentyl enol ether of 17α-hydroxypr ...
(Gestovis, Gestovister), are the 3-cyclopentyl enol ethers of 17α-hydroxyprogesterone and 17α-hydroxyprogesterone acetate, respectively, while progesterone 3-acetyl enol ether
Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed.Rao, P. N., & Edwards, B. E. (1967). ''U.S. Patent No. 3,321,495.'' Washington, DC: U.S. Pa ...
(never marketed) is the 3-acetyl enol ether of progesterone.
Although it was originally thought that progesterone ethers like quingestrone were prodrugs of progesterone, it was subsequently found that this is not the case and that quingestrone instead seems to be transformed directly into the corresponding alcohols rather than ketones. These alcohols are progesterone metabolites like pregnanolones and pregnanediols, and as some of these metabolites, for instance 3β-dihydroprogesterone, have potent progestogenic activity, this may account for the clinical efficacy of progestogen ethers like quingestrone as progestogens.
Progestogen oximes
oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted ...
conjugates of progesterone, such as progesterone carboxymethyloxime (progesterone 3-(''O''-carboxymethyl)oxime; P4-3-CMO), P1-185
P1-185, also known as progesterone 3-''O''-(L-valine)-''E''-oxime or as pregn-4-ene-3,20-dione 3-''O''-(L-valine)-''E''-oxime, is a synthetic compound, synthetic progestogen and neurosteroid and an oxime ester structural analog, analogue and prod ...
(progesterone 3-''O''-(L-valine)-''E''-oxime), EIDD-1723
EIDD-1723, also known as EPRX-01723 or as progesterone 20''E''- phosphonooxy)methyl.html"_;"title="'O''-[(phosphonooxy)methyl">'O''-[(phosphonooxy)methylximesodium_salt,_is_a_synthetic_compound.html" "title="phosphonooxy)methylxime.html" ;"title ...
(progesterone (20''E'')-20-[''O''-[(phosphonooxy)methyl]oxime] sodium salt), EIDD-036 (progesterone 20-oxime), and VOLT-02 (chemical structure unreleased), have been developed as water-soluble progesterone and neurosteroid prodrugs, although none have completed clinical development or been marketed as of yet.Guthrie, D. B., Lockwood, M. A., Natchus, M. G., Liotta, D. C., Stein, D. G., & Sayeed, I. (2017). "Progesterone phosphate analogs and uses related thereto" .Progesterone conjugate - Levolta Pharmaceuticalsspringer.com Some 19-nortestosterone progestins, including the marketed progestins norgestimate and norelgestromin and the non-marketed progestin norethisterone acetate oxime, are C3 oximes, although they have potent progestogenic activity of their own and are not necessarily prodrugs of the corresponding ketones.
See also
*List of progestogen esters
This is a list of progestogen esters, or esters of progestogens.http://www.micromedexsolutions.com
Unlike the case of testosterone and estradiol, progesterone cannot be esterified as it lacks hydroxyl groups, so all progestogen esters, with ...
* Steroid ester
* Estrogen ester
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradio ...
* Androgen ester
An androgen or anabolic steroid ester is an ester of an androgen/anabolic steroid (AAS) such as the natural testosterone or dihydrotestosterone (DHT) or the synthetic nandrolone (19-nortestosterone). Esterification renders AAS into metabolism-re ...
* List of steroid esters
List of steroid esters may refer to:
* List of androgen esters – androgen esters
* List of estrogen esters – estrogen esters
* List of progestogen esters – progestogen esters
* List of corticosteroid esters – corticosteroid esters
See al ...
* List of progestogens
References
External links
* {{Progesterone receptor modulatorsester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...