chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
in which a β-arylethylamine undergoes condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
with an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler (February 22, 1886 - August 18, 1965). Traditionally an acidic catalyst in protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labi ...
was employed with heating, however the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyze ...
. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia (). ...
, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
of the iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with a ...
ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.
The Pictet-Spengler reaction is widespread in both industry and biosynthesis. It has remained an important reaction in the fields of alkaloid
Alkaloids are a class of basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Th ...
and organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
since its inception, where it has been employed in the development of many beta-carbolines. Natural Pictet-Spengler reaction typically employ an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
, such as strictosidine synthase. Pictet-Spengler products can be isolated from many products initially derived from nature, including foodstuffs such as soy sauce and ketchup
Ketchup or catsup is a table condiment with a sweet and tangy flavor. The unmodified term ("ketchup") now typically refers to tomato ketchup, although early recipes used egg whites, mushrooms, oysters, grapes, mussels, or walnuts, among ot ...
. In such cases it is common to find the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromati ...
and various aldoses
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ...
used as the biological feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials that are feedstock for future finished products. As feedst ...
.
Nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
rings such as indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environme ...
or pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-met ...
give products in high yields and mild conditions, while less nucleophilic aromatic rings such as a phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydroge ...
group give poorer yields or require higher temperatures and strong acid. The original Pictet–Spengler reaction was the reaction of phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace am ...
and dimethoxymethane
Dimethoxymethane, also called methylal, is a colorless flammable liquid with a low boiling point, low viscosity and excellent dissolving power. It has a chloroform-like odor and a pungent taste. It is the dimethyl acetal of formaldehyde. Dimetho ...
, catalysed by hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
forming a tetrahydroisoquinoline
Tetrahydroisoquinoline (TIQ or THIQ) is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic ...
.
The Pictet–Spengler reaction has been applied to solid-phase combinatorial chemistry Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number (tens to thousands or even millions) of compounds in a single process. These compound libraries can be made as mixtures, sets of individua ...
with great success.
An analogous reaction with an aryl-β-ethanol is called oxa-Pictet–Spengler reaction
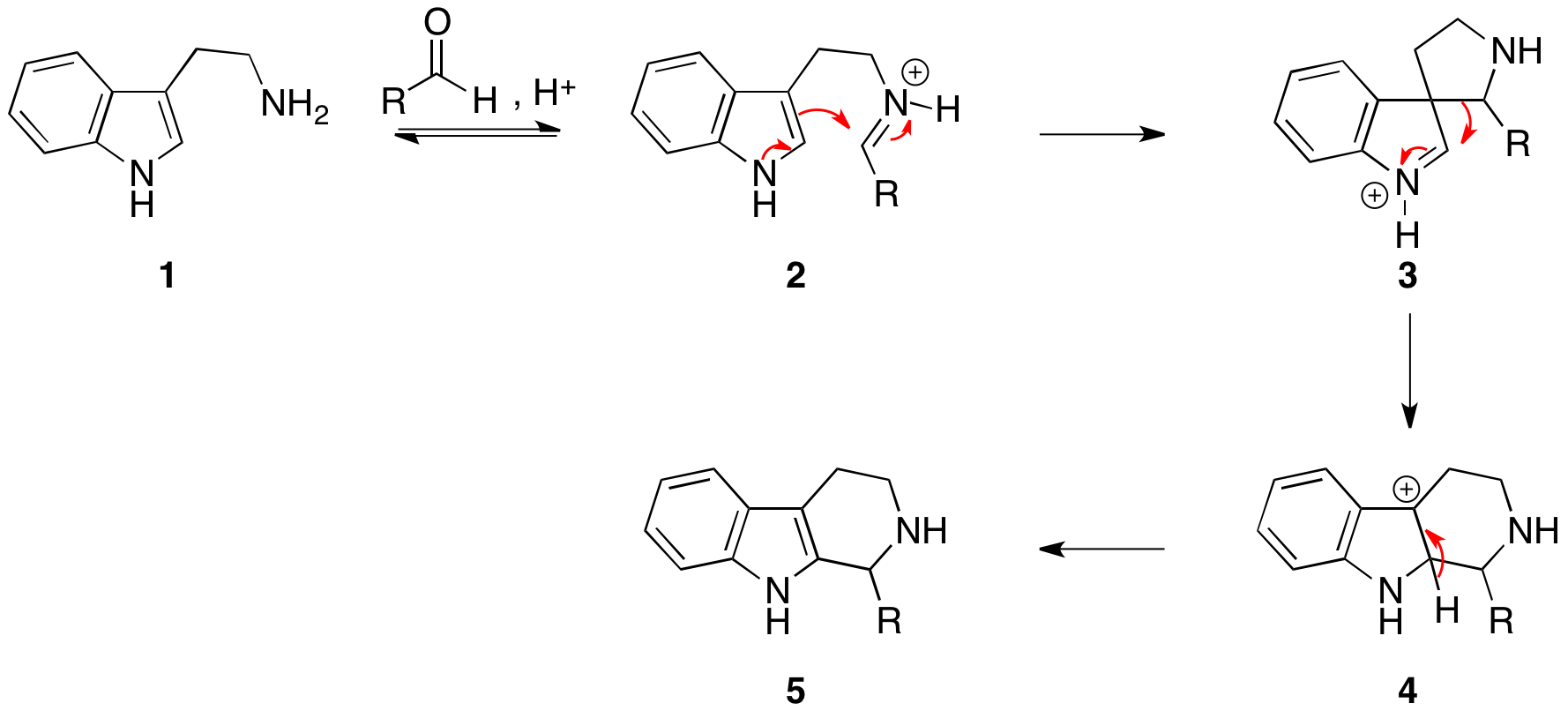
Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
occurs by initial formation of an iminium ion (2) followed by electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Chem ...
at the 3-position, in accordance with the expected nucleophilicity of indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environme ...
s, to give the spirocycle 3. After migration of the best migrating group, deprotonation gives the product (5).
Variations
Pictet–Spengler tetrahydroisoquinoline synthesis
Replacing an indole with a 3,4-dimethoxyphenyl group give the reaction named the Pictet–Spengler tetrahydroisoquinoline synthesis. Reaction conditions are generally harsher than the indole variant, and require refluxing conditions with strong acids likehydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
, trifluoroacetic acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with ...
or superacids.
''N''-acyliminium ion Pictet–Spengler reaction
Instead of catalyzing the Pictet-Spengler cyclization with strong acid, one can acylate the iminium ion forming the intermediate ''N''-acyliminium ion. The ''N''-acyliminium ion is a very powerfulelectrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
and most aromatic ring systems will cyclize under mild conditions with good yields.
Tadalafil
Tadalafil, sold under the brand name Cialis among others, is a medication used to treat erectile dysfunction (ED), benign prostatic hyperplasia (BPH), and pulmonary arterial hypertension. It is taken by mouth. Onset is typically within half a ...
is synthesized via the ''N''-acyliminium Pictet–Spengler reaction. This reaction can also be catalyzed by AuCl3 and AgOTf.
Asymmetric Pictet–Spengler reaction
When the Pictet–Spengler reaction is performed with an aldehyde other thanformaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
, a new chiral center is created. Several substrate- or auxiliary-controlled diastereoselective
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
Pictet–Spengler reactions have been developed. Additionally, List ''et al.'' have published a chiral Brønsted acid that catalyzes asymmetric Pictet–Spengler reactions.
Tryptophans: diastereocontrolled reactionThe reaction of
enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromati ...
or its short-chain alkylester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s leads to 1,2,3,4-tetrahydro- ''β''-carbolines in which a new chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
center at C-1 adopts either a '' cis'' or ''trans'' configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice boar ...
towards the C-3 carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
group. The ''cis'' conduction is kinetic
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and ente ...
ally controlled, i.e. it is performed at lower temperatures. At higher temperatures the reaction becomes reversible and usually favours racemisation. 1,3-''trans'' dominated products can be obtained with ''Nb''-benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a subst ...
ated tryptophans, which are accessible by reductive amination
Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered t ...
. The benzyl group can be removed hydrogenolytically afterwards. As a rough rule, 13C NMR signals for C1 and C3 are downfield shifted in ''cis'' products relative to ''trans'' products (see steric compression effect).
See also
* Bischler–Napieralski reaction *Pomeranz–Fritsch reaction
The Pomeranz–Fritsch reaction, also named Pomeranz–Fritsch cyclization, is a named reaction in organic chemistry. It is named after Paul Fritsch (chemist), Paul Fritsch (1859–1913) and Cäsar Pomeranz (1860–1926). In general it is a synthe ...
References
{{DEFAULTSORT:Pictet-Spengler Reaction Condensation reactions Heterocycle forming reactions Name reactions