Physical Organic Chemistry on:
[Wikipedia]
[Google]
[Amazon]
Physical organic chemistry, a term coined by
 Chemists use the study of intramolecular and intermolecular non-covalent bonding/interactions in molecules to evaluate reactivity. Such interactions include, but are not limited to,
Chemists use the study of intramolecular and intermolecular non-covalent bonding/interactions in molecules to evaluate reactivity. Such interactions include, but are not limited to,
 The properties of
The properties of
 The study of
The study of
 A modern example of the study of
A modern example of the study of
Gaussian, an example of a commercially available quantum mechanical software package used. particularly, in academic settings
accessed 21 June 2015. .g., see p. 422 for a group theoretical/symmetry description of atomic orbitals contributing to bonding in methane, CH4, and pp. 390f for estimation of π-electron binding energy for 1,3-butadiene by the Hückel method.] * Thomas H. Lowry & Kathleen Schueller Richardson, 1987, ''Mechanism and Theory in Organic Chemistry,'' 3rd Edn., New York, NY, USA:Harper & Row, , se
accessed 20 June 2015. [The authoritative textbook on the subject, containing a number of appendices that provide technical details on molecular orbital theory, kinetic isotope effects, transition state theory, and radical chemistry.] * Eric V. Anslyn & Dennis A. Dougherty, 2006, ''Modern Physical Organic Chemistry'', Sausalito, Calif.: University Science Books, . [A modernized and streamlined treatment with an emphasis on applications and cross-disciplinary connections.] *Michael B. Smith & Jerry March, 2007, "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure," 6th Ed., New York, NY, USA:Wiley & Sons, , se
accessed 19 June 2015. * Francis A. Carey & Richard J. Sundberg, 2006, "Advanced Organic Chemistry: Part A: Structure and Mechanisms," 4th Edn., New York, NY, USA:Springer Science & Business Media, , se
accessed 19 June 2015. * Hammett, Louis P. (1940) ''Physical Organic Chemistry,'' New York, NY, USA: McGraw Hill, se
accessed 20 June 2015.
accessed 22 June 2015. (This book chapter surveys a very wide range of physical properties and their estimation, including the narrow list of thermochemical properties appearing in the June 2015 WP article, placing the Benson et al. method alongside many other methods. L. K. Doraiswamy is ''Anson Marston Distinguished Professor of Engineering'' at
Louis Hammett
Louis Plack Hammett (April 7, 1894 – February 9, 1987) was an American physical chemist. He is known for the Hammett equation, which relates reaction rates to equilibrium constants for certain classes of organic reactions involving subst ...
in 1940, refers to a discipline of organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
that focuses on the relationship between chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of at ...
s and reactivity, in particular, applying experimental tools of physical chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mecha ...
to the study of organic molecules. Specific focal points of study include the rates of organic reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
, the relative chemical stabilities of the starting materials, reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s, transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
s, and products of chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s, and non-covalent aspects of solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the ...
and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
and rate for each organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
of interest.
Application
Physical organic chemists usetheoretical
A theory is a rational type of abstract thinking about a phenomenon, or the results of such thinking. The process of contemplative and rational thinking is often associated with such processes as observational study or research. Theories may be ...
and experimental approaches work to understand these foundational problems in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, including classical and statistical thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
calculations, quantum mechanical theory and computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of m ...
, as well as experimental spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
(e.g., NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
), spectrometry (e.g., MS), and crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
approaches. The field therefore has applications to a wide variety of more specialized fields, including electro- and photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
, polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
and supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
, and bioorganic chemistry Bioorganic chemistry is a scientific discipline that combines organic chemistry and biochemistry. It is that branch of life science that deals with the study of biological processes using chemical methods. Protein and enzyme function are examples of ...
, enzymology
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
, and chemical biology
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and ma ...
, as well as to commercial enterprises involving process chemistry
Process chemistry is the arm of pharmaceutical chemistry concerned with the development and optimization of a synthetic scheme and pilot plant procedure to manufacture compounds for the drug development phase. Process chemistry is distinguished fr ...
, chemical engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials int ...
, materials science and nanotechnology
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal o ...
, and pharmacology
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemica ...
in drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.
Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by ...
by design.
Scope
Physical organic chemistry is the study of the relationship between structure and reactivity of organic molecules. More specifically, physical organic chemistry applies the experimental tools ofphysical chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mecha ...
to the study of the structure of organic molecules and provides a theoretical framework that interprets how structure influences both mechanisms and rates of organic reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
. It can be thought of as a subfield that bridges organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
with physical chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mecha ...
.
Physical organic chemists use both experimental and theoretical disciplines such as spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
, spectrometry, crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
, computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of m ...
, and quantum theory
Quantum theory may refer to:
Science
*Quantum mechanics, a major field of physics
*Old quantum theory, predating modern quantum mechanics
* Quantum field theory, an area of quantum mechanics that includes:
** Quantum electrodynamics
** Quantum ...
to study both the rates of organic reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
and the relative chemical stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system.
Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibri ...
of the starting materials, transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
s, and products. Chemists in this field work to understand the physical underpinnings of modern organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, and therefore physical organic chemistry has applications in specialized areas including polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are ...
, supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
, electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, and photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
.
History
The term ''physical organic chemistry'' was itself coined byLouis Hammett
Louis Plack Hammett (April 7, 1894 – February 9, 1987) was an American physical chemist. He is known for the Hammett equation, which relates reaction rates to equilibrium constants for certain classes of organic reactions involving subst ...
in 1940 when he used the phrase as a title for his textbook.
Chemical structure and thermodynamics
Thermochemistry
Organic chemists use the tools ofthermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
to study the bonding, stability
Stability may refer to:
Mathematics
*Stability theory, the study of the stability of solutions to differential equations and dynamical systems
** Asymptotic stability
** Linear stability
** Lyapunov stability
** Orbital stability
** Structural sta ...
, and energetics of chemical systems. This includes experiments to measure or determine the enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
(ΔH), entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
(ΔS), and Gibbs' free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
(ΔG) of a reaction, transformation, or isomerization. Chemists may use various chemical and mathematical analyses, such as a Van 't Hoff plot, to calculate these values.
Empirical constants such as bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
, standard heat of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a Chemical compound, compound is the change of enthalpy during the formation of 1 mole (unit), mole of the substance from its constituent Chemical ...
(ΔHf°), and heat of combustion (ΔHc°) are used to predict the stability of molecules and the change in enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
(ΔH) through the course of the reactions. For complex molecules, a ΔHf° value may not be available but can be estimated using molecular fragments with known heats of formation. This type of analysis is often referred to as Benson group increment theory Benson may refer to:
Animals
*Benson (fish), largest common carp caught in Britain
Places Geography
Canada
*Rural Municipality of Benson No. 35, Saskatchewan; rural municipality
*Benson, Saskatchewan; hamlet
United Kingdom
*Benson, Oxfordshire
...
, after chemist Sidney Benson who spent a career developing the concept.
The thermochemistry of reactive intermediates—carbocations
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
, carbanions
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
, and radicals—is also of interest to physical organic chemists. Group increment data are available for radical systems. Carbocation and carbanion stabilities can be assessed using hydride ion affinities and pKa values, respectively.
Conformational analysis
One of the primary methods for evaluating chemical stability and energetics isconformational analysis
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mo ...
. Physical organic chemists use conformational analysis to evaluate the various types of strain
Strain may refer to:
Science and technology
* Strain (biology), variants of plants, viruses or bacteria; or an inbred animal used for experimental purposes
* Strain (chemistry), a chemical stress of a molecule
* Strain (injury), an injury to a mu ...
present in a molecule to predict reaction products. Strain can be found in both acyclic and cyclic molecules, manifesting itself in diverse systems as torsional strain, allylic strain
250 px , right , Allylic strain in an olefin.
Allylic strain (also known as A1,3 strain, 1,3-allylic strain, or A-strain) in organic chemistry is a type of strain energy resulting from the interaction between a substituent on one end of an olef ...
, ring strain, and ''syn''-pentane strain. A-values provide a quantitative basis for predicting the conformation of a substituted cyclohexane, an important class of cyclic organic compounds whose reactivity is strongly guided by conformational effects. The A-value is the difference in the Gibbs' free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
between the axial and equatorial forms of substituted cyclohexane, and by adding together the A-values of various substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
s it is possible to quantitatively predict the preferred conformation of a cyclohexane derivative.
In addition to molecular stability, conformational analysis is used to predict reaction products. One commonly cited example of the use of conformational analysis
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mo ...
is a bi-molecular elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
(E2). This reaction proceeds most readily when the nucleophile attacks the species that is antiperiplanar
In organic chemistry, anti-periplanar, or antiperiplanar, describes the bond angle in a molecule. In this conformer, the dihedral angle of the bond and the bond is greater than +150° or less than −150° (Figures 1 and 2). Anti-periplanar i ...
to the leaving group. A molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
analysis of this phenomenon suggest that this conformation provides the best overlap between the electrons in the R-H σ bonding orbital
In theoretical chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule. In MO theory, electrons are portrayed to move in waves. ...
that is undergoing nucleophilic attack and the empty σ* antibonding orbital of the R-X bond that is being broken. By exploiting this effect, conformational analysis
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mo ...
can be used to design molecules that possess enhanced reactivity.
The physical processes which give rise to bond rotation barriers are complex, and these barriers have been extensively studied through experimental and theoretical methods. A number of recent articles have investigated the predominance of the steric, electrostatic
Electrostatics is a branch of physics that studies electric charges at rest (static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber ...
, and hyperconjugative contributions to rotational barriers in ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petr ...
, butane
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name but ...
, and more substituted molecules.
Non-covalent interactions
hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
, electrostatic interactions
Electrostatics is a branch of physics that studies electric charges at Rest (physics), rest (static electricity).
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles af ...
between charged molecules, dipole-dipole interactions
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
, polar-π and cation-π interactions, π-stacking, donor-acceptor chemistry, and halogen bonding A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bo ...
. In addition, the hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
—the association of organic compounds in water—is an electrostatic, non-covalent interaction
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The c ...
of interest to chemists. The precise physical origin of the hydrophobic effect originates from many complex interactions, but it is believed to be the most important component of biomolecular recognition in water. For example, Xu and Melcher ''et al.'' elucidated the structural basis for folic acid recognition by folate acid receptor proteins. The strong interaction between folic acid
Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and ...
and folate receptor
Folate receptors bind folate and reduced folic acid derivatives and mediates delivery of tetrahydrofolate to the interior of cells. It is then converted from monoglutamate to polyglutamate forms - such as 5-methyltetrahydrofolate - as only monog ...
was attributed to both hydrogen bonds and hydrophobic interactions
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water#Properties, water molecules. The word hydrophobic literally means "water-fearing", and it describes the Segregation in m ...
. The study of non-covalent interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The c ...
is also used to study binding and cooperativity in supramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
assemblies and macrocyclic compounds such as crown ethers
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
and cryptands, which can act as hosts to guest molecules.
Acid–base chemistry
acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s and bases are relevant to physical organic chemistry. Organic chemists are primarily concerned with Brønsted–Lowry acids/bases as proton donors/acceptors and Lewis acids/bases as electron acceptors/donors in organic reactions. Chemists use a series of factors developed from physical chemistry -- electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
/Induction
Induction, Inducible or Inductive may refer to:
Biology and medicine
* Labor induction (birth/pregnancy)
* Induction chemotherapy, in medicine
* Induced stem cells, stem cells derived from somatic, reproductive, pluripotent or other cell t ...
, bond strengths, resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
, hybridization, aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturate ...
, and solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the ...
—to predict relative acidities and basicities.
The hard/soft acid/base principle is utilized to predict molecular interactions and reaction direction. In general, interactions between molecules of the same type are preferred. That is, hard acids will associate with hard bases, and soft acids with soft bases. The concept of hard acids and bases is often exploited in the synthesis of inorganic coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es.
Kinetics
Physical organic chemists use the mathematical foundation of chemical kinetics to study the rates of reactions and reaction mechanisms. Unlike thermodynamics, which is concerned with the relative stabilities of the products and reactants (ΔG°) and their equilibrium concentrations, the study of kinetics focuses on the free energy of activation (ΔG‡) -- the difference in free energy between the reactant structure and the transition state structure—of a reaction, and therefore allows a chemist to study the process of equilibration. Mathematically derived formalisms such as theHammond Postulate
Hammond's postulate (or alternatively the Hammond–Leffler postulate), is a hypothesis in physical organic chemistry which describes the geometric structure of the transition state in an organic chemical reaction. First proposed by George Hammon ...
, the Curtin-Hammett principle, and the theory of microscopic reversibility are often applied to organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
. Chemists have also used the principle of thermodynamic versus kinetic control to influence reaction products.
Rate laws
The study ofchemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in wh ...
is used to determine the rate law
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial react ...
for a reaction. The rate law provides a quantitative relationship between the rate of a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
and the concentrations
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', ''number concentration'', a ...
or pressures
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
of the chemical species present. Note, Amazon rather than Google allows access into this text. Rate laws must be determined by experimental measurement and generally cannot be elucidated from the chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
. The experimentally determined rate law refers to the stoichiometry of the transition state structure relative to the ground state structure. Determination of the rate law was historically accomplished by monitoring the concentration of a reactant during a reaction through gravimetric analysis
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been ...
, but today it is almost exclusively done through fast and unambiguous spectroscopic
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
techniques. In most cases, the determination of rate equations is simplified by adding a large excess ("flooding") all but one of the reactants.
Catalysis
 The study of
The study of catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
and catalytic reactions is very important to the field of physical organic chemistry. A catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
participates in the chemical reaction but is not consumed in the process. A catalyst lowers the activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
barrier (ΔG‡), increasing the rate of a reaction by either stabilizing the transition state structure or destabilizing a key reaction intermediate, and as only a small amount of catalyst is required it can provide economic access to otherwise expensive or difficult to synthesize organic molecules. Catalysts may also influence a reaction rate by changing the mechanism
Mechanism may refer to:
* Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that ...
of the reaction.
Kinetic isotope effect
Although a rate law provides the stoichiometry of thetransition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
structure, it does not provide any information about breaking or forming bonds. The substitution of an isotope near a reactive position often leads to a change in the rate of a reaction. Isotopic substitution changes the potential energy of reaction intermediates and transition states because heavier isotopes form stronger bonds with other atoms. Atomic mass affects the zero-point vibrational state of the associated molecules, shorter and stronger bonds in molecules with heavier isotopes and longer, weaker bonds in molecules with light isotopes. Because vibrational motions will often change during a course of a reaction, due to the making and breaking of bonds, the frequencies will be affected, and the substitution of an isotope can provide insight into the reaction mechanism and rate law.
Substituent effects
The study of how substituents affect the reactivity of a molecule or the rate of reactions is of significant interest to chemists. Substituents can exert an effect through both steric and electronic interactions, the latter of which includeresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
and inductive effects. The polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementar ...
of molecule can also be affected. Most substituent effects are analyzed through linear free energy relationships (LFERs). The most common of these is the Hammett Plot Analysis. This analysis compares the effect of various substituents on the ionization of benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
with their impact on diverse chemical systems. The parameters of the Hammett plots are sigma (σ) and rho (ρ). The value of σ indicates the acidity of substituted benzoic acid relative to the unsubstituted form. A positive σ value indicates the compound is more acidic, while a negative value indicates that the substituted version is less acidic. The ρ value is a measure of the sensitivity of the reaction to the change in substituent, but only measures inductive effects. Therefore, two new scales were produced that evaluate the stabilization of localized charge through resonance. One is σ+, which concerns substituents that stabilize positive charges via resonance, and the other is σ− which is for groups that stabilize negative charges via resonance. Hammett analysis can be used to help elucidate the possible mechanisms of a reaction. For example, if it is predicted that the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
structure has a build-up of negative charge relative to the ground state structure, then electron-donating groups would be expected to increase the rate of the reaction.
Other LFER scales have been developed. Steric and polar effects are analyzed through Taft Parameters. Changing the solvent instead of the reactant can provide insight into changes in charge during the reaction. The Grunwald-Winstein Plot provides quantitative insight into these effects.
Solvent effects
Solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
can have a powerful effect on solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
, stability
Stability may refer to:
Mathematics
*Stability theory, the study of the stability of solutions to differential equations and dynamical systems
** Asymptotic stability
** Linear stability
** Lyapunov stability
** Orbital stability
** Structural sta ...
, and reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
. A change in solvent can also allow a chemist to influence the thermodynamic or kinetic control of the reaction. Reactions proceed at different rates in different solvents due to the change in charge distribution during a chemical transformation. Solvent effects may operate on the ground state and/or transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
structures.
An example of the effect of solvent on organic reactions is seen in the comparison of SN1 and SN2 reactions.
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
can also have a significant effect on the thermodynamic equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In the ...
of a system, for instance as in the case of keto-enol tautomerizations. In non-polar aprotic solvents, the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The t ...
form is strongly favored due to the formation of an intramolecular hydrogen-bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
, while in polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
aprotic A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydro ...
solvents, such as methylene chloride
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
, the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The t ...
form is less favored due to the interaction between the polar solvent and the polar diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
. In protic
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile ...
solvents, the equilibrium lies towards the keto form as the intramolecular hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
competes with hydrogen bonds originating from the solvent.
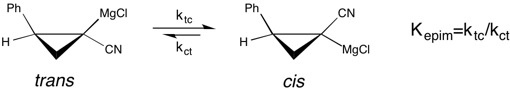
 A modern example of the study of
A modern example of the study of solvent effects
In chemistry, solvent effects are the influence of a solvent on chemical reactivity or molecular associations. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic a ...
on chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the sy ...
can be seen in a study of the epimerization
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is t ...
of chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
cyclopropylnitrile Grignard reagents
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
. This study reports that the equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for the ''cis'' to ''trans'' isomerization of the Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
is much greater—the preference for the ''cis'' form is enhanced—in THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
as a reaction solvent, over diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
. However, the faster rate of '' cis-trans isomerization'' in THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
results in a loss of stereochemical
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
purity. This is a case where understanding the effect of solvent on the stability of the molecular configuration The molecular configuration of a molecule is the ''permanent'' geometry that results from the spatial arrangement of its bonds. The ability of the same set of atoms to form two or more molecules with different configurations is stereoisomerism. ...
of a reagent is important with regard to the selectivity observed in an asymmetric synthesis.
Quantum chemistry
Many aspects of the structure-reactivity relationship in organic chemistry can be rationalized throughresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
, electron pushing, induction
Induction, Inducible or Inductive may refer to:
Biology and medicine
* Labor induction (birth/pregnancy)
* Induction chemotherapy, in medicine
* Induced stem cells, stem cells derived from somatic, reproductive, pluripotent or other cell t ...
, the eight electron rule, and s-p hybridization, but these are only helpful formalisms and do not represent physical reality. Due to these limitations, a true understanding of physical organic chemistry requires a more rigorous approach grounded in particle physics
Particle physics or high energy physics is the study of fundamental particles and forces that constitute matter and radiation. The fundamental particles in the universe are classified in the Standard Model as fermions (matter particles) an ...
. Quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
provides a rigorous theoretical framework capable of predicting the properties of molecules through calculation of a molecule's electronic structure, and it has become a readily available tool in physical organic chemists in the form of popular software packages. The power of quantum chemistry is built on the wave model of the atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
, in which the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
is a very small, positively charged sphere surrounded by a diffuse electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
cloud. Particles are defined by their associated wavefunction
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements ...
, an equation which contains all information associated with that particle. All information about the system is contained in the wavefunction. This information is extracted from the wavefunction
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements ...
through the use of mathematical operators.
The energy associated with a particular wavefunction
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements ...
, perhaps the most important information contained in a wavefunction, can be extracted by solving the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the ...
(above, Ψ is the wavefunction, E is the energy, and Ĥ is the Hamiltonian operator) in which an appropriate Hamiltonian operator
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonia ...
is applied. In the various forms of the Schrödinger equation, the overall size of a particle's probability distribution increases with decreasing particle mass. For this reason, nuclei are of negligible size in relation to much lighter electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
and are treated as point charges in practical applications of quantum chemistry.
Due to complex interactions which arise from electron-electron repulsion, algebraic solutions of the Schrödinger equation are only possible for systems with one electron such as the hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
atom, H2+, H32+, etc.; however, from these simple models arise all the familiar atomic (s,p,d,f) and bonding (σ,π) orbitals. In systems with multiple electrons, an overall multielectron wavefunction describes all of their properties at once. Such wavefunctions are generated through the linear addition of single electron wavefunctions to generate an initial guess, which is repeatedly modified until its associated energy is minimized. Thousands of guesses are often required until a satisfactory solution is found, so such calculations are performed by powerful computers. Importantly, the solutions for atoms with multiple electrons give properties such as diameter and electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
which closely mirror experimental data and the patterns found in the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. The solutions for molecules, such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
, provide exact representations of their electronic structure
In quantum chemistry, electronic structure is the state of motion of electrons in an electrostatic field created by stationary nuclei. The term encompasses both the wave functions of the electrons and the energies associated with them. Electro ...
which are unobtainable by experimental methods. Instead of four discrete σ-bonds from carbon to each hydrogen atom, theory predicts a set of four bonding molecular orbitals which are delocalized across the entire molecule. Similarly, the true electronic structure of 1,3-butadiene shows delocalized π-bonding molecular orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
stretching through the entire molecule rather than two isolated double bonds as predicted by a simple Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the chemical bonding, bonding between atoms of a molecule, as well as the lone pairs ...
.
A complete electronic structure offers great predictive power for organic transformations and dynamics, especially in cases concerning aromatic molecules, extended π systems, bonds between metal ions and organic molecules, molecules containing nonstandard heteroatoms
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
like selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
and boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
, and the conformational dynamics of large molecules such as proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
wherein the many approximations in chemical formalisms make structure and reactivity prediction impossible. An example of how electronic structure determination is a useful tool for the physical organic chemist is the metal-catalyzed dearomatization of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
. Chromium tricarbonyl is highly electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
due to the withdrawal of electron density from filled chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
d-orbitals into antibonding CO orbitals, and is able to covalently bond to the face of a benzene molecule through delocalized molecular orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
. The CO ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
inductively draw electron density from benzene through the chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
atom, and dramatically activate benzene to nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
attack. Nucleophiles are then able to react to make hexacyclodienes, which can be used in further transformations such as Diels Alder cycloadditons.
Quantum chemistry can also provide insight into the mechanism of an organic transformation without the collection of any experimental data. Because wavefunctions provide the total energy of a given molecular state, guessed molecular geometries can be optimized to give relaxed molecular structures very similar to those found through experimental methods. Reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecu ...
s can then be simulated, and transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
structures solved. Solving a complete energy surface for a given reaction is therefore possible, and such calculations have been applied to many problems in organic chemistry where kinetic data is unavailable or difficult to acquire.
Spectroscopy, spectrometry, and crystallography
Physical organic chemistry often entails the identification of molecular structure, dynamics, and the concentration of reactants in the course of a reaction. The interaction of molecules with light can afford a wealth of data about such properties through nondestructive spectroscopic experiments, withlight
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 tera ...
absorbed when the energy of a photon matches the difference in energy between two states in a molecule and emitted when an excited state in a molecule collapses to a lower energy state. Spectroscopic techniques are broadly classified by the type of excitation being probed, such as vibrational, rotational
Rotation, or spin, is the circular movement of an object around a '' central axis''. A two-dimensional rotating object has only one possible central axis and can rotate in either a clockwise or counterclockwise direction. A three-dimensional ...
, electronic
Electronic may refer to:
*Electronics, the science of how to control electric energy in semiconductor
* ''Electronics'' (magazine), a defunct American trade journal
*Electronic storage, the storage of data using an electronic device
*Electronic co ...
, nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
(NMR), and electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
spectroscopy. In addition to spectroscopic data, structure determination is often aided by complementary data collected from X-Ray diffraction and mass spectrometric experiments.
NMR and EPR spectroscopy
One of the most powerful tools in physical organic chemistry isNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
. An external magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
applied to a paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, d ...
nucleus generates two discrete states, with positive and negative spin values diverging in energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
; the difference in energy can then be probed by determining the frequency of light needed to excite a change in spin state for a given magnetic field. Nuclei that are not indistinguishable in a given molecule absorb at different frequencies, and the integrated peak area in an NMR spectrum is proportional to the number of nuclei responding to that frequency. It is possible to quantify the relative concentration of different organic molecules simply by integration
Integration may refer to:
Biology
*Multisensory integration
*Path integration
* Pre-integration complex, viral genetic material used to insert a viral genome into a host genome
*DNA integration, by means of site-specific recombinase technology, ...
peaks in the spectrum, and many kinetic experiments can be easily and quickly performed by following the progress of a reaction within one NMR sample. Proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the struc ...
is often used by the synthetic organic chemist because protons associated with certain functional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
give characteristic absorption energies, but NMR spectroscopy can also be performed on isotopes
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
, boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
, and a host of other elements. In addition to simple absorption experiments, it is also possible to determine the rate of fast atom exchange reactions through suppression exchange measurements, interatomic distances through multidimensional nuclear overhauser effect The nuclear Overhauser effect (NOE) is the transfer of nuclear spin polarization from one population of spin-active nuclei (e.g. 1H, 13C, 15N etc.) to another via cross-relaxation. A phenomenological definition of the NOE in nuclear magnetic res ...
experiments, and through-bond spin-spin coupling through homonuclear correlation spectroscopy. In addition to the spin excitation properties of nuclei, it is also possible to study the properties of organic radicals through the same fundamental technique. Unpaired electrons also have a net spin, and an external magnetic field allows for the extraction of similar information through electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
(EPR) spectroscopy.
Vibrational spectroscopy
Vibrational spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, or infrared (IR) spectroscopy, allows for the identification of functional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
and, due to its low expense and robustness, is often used in teaching labs and the real-time monitoring of reaction progress in difficult to reach environments (high pressure, high temperature, gas phase, phase boundaries). Molecular vibrations
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 ...
are quantized in an analogous manner to electronic wavefunctions, with integer increases in frequency leading to higher energy states. The difference in energy between vibrational states is nearly constant, often falling in the energy range corresponding to infrared photons, because at normal temperatures molecular vibrations closely resemble harmonic oscillators
In classical mechanics, a harmonic oscillator is a system that, when displaced from its equilibrium position, experiences a restoring force ''F'' proportional to the displacement ''x'':
\vec F = -k \vec x,
where ''k'' is a positive consta ...
. It allows for the crude identification of functional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
in organic molecules, but spectra are complicated by vibrational coupling between nearby functional groups in complex molecules. Therefore, its utility in structure determination is usually limited to simple molecules. Further complicating matters is that some vibrations do not induce a change in the molecular dipole moment
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
and will not be observable with standard IR absorption spectroscopy. These can instead be probed through Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman sp ...
, but this technique requires a more elaborate apparatus and is less commonly performed. However, as Raman spectroscopy relies on light scattering it can be performed on microscopic samples such as the surface of a heterogeneous catalyst
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. P ...
, a phase boundary, or on a one microliter (µL) subsample within a larger liquid volume. The applications of vibrational spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
are often used by astronomers to study the composition of molecular gas clouds, extrasolar planetary atmospheres, and planetary surfaces.
Electronic excitation spectroscopy
Electronic excitation spectroscopy, or ultraviolet-visible (UV-vis) spectroscopy, is performed in thevisible
Visibility, in meteorology, is a measure of the distance at which an object or light can be seen.
Visibility may also refer to:
* A measure of turbidity in water quality control
* Interferometric visibility, which quantifies interference contrast ...
and ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
regions of the electromagnetic spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from ...
and is useful for probing the difference in energy between the highest energy occupied (HOMO) and lowest energy unoccupied (LUMO) molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
s. This information is useful to physical organic chemists in the design of organic photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
systems and dyes, as absorption of different wavelengths of visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
give organic molecules color. A detailed understanding of an electronic structure is therefore helpful in explaining electronic excitations, and through careful control of molecular structure it is possible to tune the HOMO-LUMO gap to give desired colors and excited state properties.
Mass spectrometry
Mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
is a technique which allows for the measurement of molecular mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quanti ...
and offers complementary data to spectroscopic
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
techniques for structural identification. In a typical experiment a gas phase sample of an organic material
Organic matter, organic material, or natural organic matter refers to the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have c ...
is ionized
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
and the resulting ionic species are accelerated by an applied electric field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field fo ...
into a magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
. The deflection imparted by the magnetic field, often combined with the time it takes for the molecule to reach a detector, is then used to calculate the mass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different elementar ...
of the molecule. Often in the course of sample ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
large molecules break apart, and the resulting data show a parent mass and a number of smaller fragment masses; such fragmentation can give rich insight into the sequence of proteins and nucleic acid polymers. In addition to the mass of a molecule and its fragments, the distribution of isotopic variant masses can also be determined and the qualitative presence of certain elements identified due to their characteristic natural isotope distribution. The ratio of fragment mass population to the parent ion population can be compared against a library of empirical fragmentation data and matched to a known molecular structure. Combined gas chromatography and mass spectrometry is used to qualitatively identify molecules and quantitatively measure concentration with great precision and accuracy, and is widely used to test for small quantities of biomolecules and illicit narcotics in blood samples. For synthetic organic chemists it is a useful tool for the characterization of new compounds and reaction products.
Crystallography
Unlike spectroscopic methods,X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
always allows for unambiguous structure determination and provides precise bond angles and lengths totally unavailable through spectroscopy. It is often used in physical organic chemistry to provide an absolute molecular configuration The molecular configuration of a molecule is the ''permanent'' geometry that results from the spatial arrangement of its bonds. The ability of the same set of atoms to form two or more molecules with different configurations is stereoisomerism. ...
and is an important tool in improving the synthesis of a pure enantiomeric
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
substance. It is also the only way to identify the position and bonding of elements that lack an NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
active nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
such as oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
. Indeed, before x-ray structural determination methods were made available in the early 20th century all organic structures were entirely conjectural: tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, for example, was only confirmed by the crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
of diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of car ...
, and the delocalized structure of benzene was confirmed by the crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
of hexamethylbenzene
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each ...
. While crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
provides organic chemists with highly satisfying data, it is not an everyday technique in organic chemistry because a perfect single crystal
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries.RIWD. "Re ...
of a target compound must be grown. Only complex molecules, for which NMR data cannot be unambiguously interpreted, require this technique. In the example below, the structure of the host–guest complex would have been quite difficult to solve without a single crystal structure: there are no protons on the fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
, and with no covalent bonds between the two halves of the organic complex spectroscopy alone was unable to prove the hypothesized structure.
See also
* ''Journal of Physical Organic Chemistry
The ''Journal of Physical Organic Chemistry'' is a monthly peer-reviewed scientific journal, published since 1988 by John Wiley & Sons. It covers research in physical organic chemistry in its broadest sense and is available both online and in print ...
''
Gaussian, an example of a commercially available quantum mechanical software package used. particularly, in academic settings
References
Further reading
General
* Peter Atkins & Julio de Paula, 2006, "Physical chemistry," 8th Edn., New York, NY, USA:Macmillan, , seaccessed 21 June 2015. .g., see p. 422 for a group theoretical/symmetry description of atomic orbitals contributing to bonding in methane, CH4, and pp. 390f for estimation of π-electron binding energy for 1,3-butadiene by the Hückel method.] * Thomas H. Lowry & Kathleen Schueller Richardson, 1987, ''Mechanism and Theory in Organic Chemistry,'' 3rd Edn., New York, NY, USA:Harper & Row, , se
accessed 20 June 2015. [The authoritative textbook on the subject, containing a number of appendices that provide technical details on molecular orbital theory, kinetic isotope effects, transition state theory, and radical chemistry.] * Eric V. Anslyn & Dennis A. Dougherty, 2006, ''Modern Physical Organic Chemistry'', Sausalito, Calif.: University Science Books, . [A modernized and streamlined treatment with an emphasis on applications and cross-disciplinary connections.] *Michael B. Smith & Jerry March, 2007, "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure," 6th Ed., New York, NY, USA:Wiley & Sons, , se
accessed 19 June 2015. * Francis A. Carey & Richard J. Sundberg, 2006, "Advanced Organic Chemistry: Part A: Structure and Mechanisms," 4th Edn., New York, NY, USA:Springer Science & Business Media, , se
accessed 19 June 2015. * Hammett, Louis P. (1940) ''Physical Organic Chemistry,'' New York, NY, USA: McGraw Hill, se
accessed 20 June 2015.
History
* n outstanding starting point on the history of the field, from a critically important contributor, referencing and discussing the early Hammett text, etc.Thermochemistry
* L. K. Doraiswamy, 2005, "Estimation of properties of organic compounds (Ch. 3)," pp. 36–51, 118-124 (refs.), in ''Organic Synthesis Engineering,'' Oxford, Oxon, ENG:Oxford University Press, , seaccessed 22 June 2015. (This book chapter surveys a very wide range of physical properties and their estimation, including the narrow list of thermochemical properties appearing in the June 2015 WP article, placing the Benson et al. method alongside many other methods. L. K. Doraiswamy is ''Anson Marston Distinguished Professor of Engineering'' at
Iowa State University
Iowa State University of Science and Technology (Iowa State University, Iowa State, or ISU) is a public land-grant research university in Ames, Iowa. Founded in 1858 as the Iowa Agricultural College and Model Farm, Iowa State became one of the n ...
.)
*
{{DEFAULTSORT:Physical Organic Chemistry
Organic chemistry