Phosphorus(V) Chloride on:
[Wikipedia]
[Google]
[Amazon]
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.
 In solutions of polar solvents, PCl5 undergoes self- ionization. Dilute solutions dissociate according to the following equilibrium:
:PCl5 + Cl−
At higher concentrations, a second equilibrium becomes more prevalent:
:2 PCl5 +
The cation and the anion are tetrahedral and octahedral, respectively. At one time, PCl5 in solution was thought to form a dimeric structure, P2Cl10, but this suggestion is not supported by Raman spectroscopic measurements.
In solutions of polar solvents, PCl5 undergoes self- ionization. Dilute solutions dissociate according to the following equilibrium:
:PCl5 + Cl−
At higher concentrations, a second equilibrium becomes more prevalent:
:2 PCl5 +
The cation and the anion are tetrahedral and octahedral, respectively. At one time, PCl5 in solution was thought to form a dimeric structure, P2Cl10, but this suggestion is not supported by Raman spectroscopic measurements.
 It also converts
It also converts
The period 3 chlorides
{{Authority control Phosphorus chlorides Hypervalent molecules Phosphorus(V) compounds
Structure
The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (''D''3h)symmetry
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definit ...
. The hypervalent nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbitals ( molecular orbital theory) or resonance (valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
). This trigonal bipyramidal structure persists in nonpolar solvents, such as CS2 and CCl4. In the solid state PCl5 is an ionic compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged i ...
, formulated .
 In solutions of polar solvents, PCl5 undergoes self- ionization. Dilute solutions dissociate according to the following equilibrium:
:PCl5 + Cl−
At higher concentrations, a second equilibrium becomes more prevalent:
:2 PCl5 +
The cation and the anion are tetrahedral and octahedral, respectively. At one time, PCl5 in solution was thought to form a dimeric structure, P2Cl10, but this suggestion is not supported by Raman spectroscopic measurements.
In solutions of polar solvents, PCl5 undergoes self- ionization. Dilute solutions dissociate according to the following equilibrium:
:PCl5 + Cl−
At higher concentrations, a second equilibrium becomes more prevalent:
:2 PCl5 +
The cation and the anion are tetrahedral and octahedral, respectively. At one time, PCl5 in solution was thought to form a dimeric structure, P2Cl10, but this suggestion is not supported by Raman spectroscopic measurements.
Related pentachlorides
AsCl5 and SbCl5 also adopt trigonal bipyramidal structures. The relevant bond distances are 211 pm (As−Cleq), 221 pm (As−Clax), 227 pm (Sb−Cleq), and 233.3 pm (Sb−Clax). At low temperatures, SbCl5 converts to the dimer, dioctahedral Sb2Cl10, structurally related toniobium pentachloride
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl ...
.
Preparation
PCl5 is prepared by the chlorination of PCl3. This reaction is used to produce around 10,000 tonnes of PCl5 per year (as of 2000). :PCl3 + Cl2 PCl5 (Δ''H'' = −124 kJ/mol) PCl5 exists in equilibrium with PCl3 and chlorine, and at 180 °C the degree of dissociation is about 40%. Because of this equilibrium, samples of PCl5 often contain chlorine, which imparts a greenish coloration.Reactions
Hydrolysis
In its most characteristic reaction, PCl5reacts
''React'' (from Spanish: ''Reacciona'') is a book by Rosa María Artal published in Spain in 2011 by Aguilar, which compiles articles by José Luis Sampedro, Baltasar Garzón, Federico Mayor Zaragoza, Javier Pérez de Albéniz, Javier López Facal ...
upon contact with water to release hydrogen chloride and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride:
:PCl5 + H2O → POCl3 + 2 HCl
In hot water, hydrolysis proceeds completely to orthophosphoric acid:
:PCl5 + 4 H2O → H3PO4 + 5 HCl
Lewis acidity
Phosphorus pentachloride is a Lewis acid. This property underpins many of its characteristic reactions, autoionization, chlorinations, hydrolysis. A well studied adduct is PCl5(pyridine).Chlorination of organic compounds
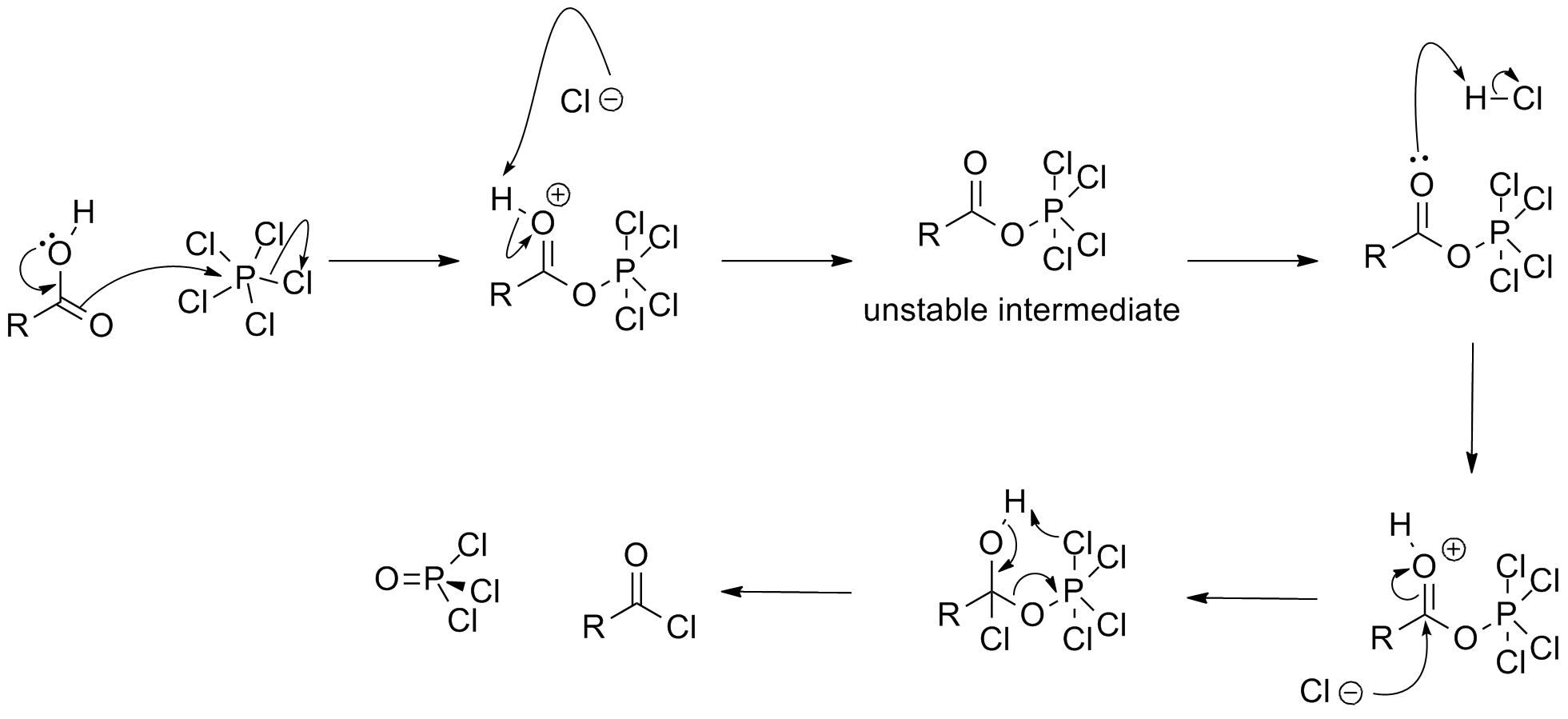
In synthetic chemistry, two classes of chlorination are usually of interest: oxidative chlorinations and substitutive chlorinations. Oxidative chlorinations entail the transfer of Cl2 from the reagent to the substrate. Substitutive chlorinations entail replacement of O or OH groups with chloride. PCl5 can be used for both processes. Upon treatment with PCl5,carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s convert to the corresponding acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
. The following mechanism has been proposed:
: It also converts
It also converts alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s to alkyl chlorides. Thionyl chloride is more commonly used in the laboratory because the resultant sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
is more easily separated from the organic products than is POCl3.
PCl5 reacts with a tertiary amides, such as dimethylformamide (DMF), to give dimethylchloromethyleneammonium chloride, which is called the Vilsmeier reagent, CH3)2N=CClHl. More typically, a related salt is generated from the reaction of DMF and POCl3. Such reagents are useful in the preparation of derivatives of benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
by formylation and for the conversion of C−OH groups into C−Cl groups.
It is especially renowned for the conversion of C=O groups to CCl2 groups. For example, benzophenone and phosphorus pentachloride react to give the diphenyldichloromethane:
:(C6H5)2CO + PCl5 → (C6H5)2CCl2 + POCl3
The electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
character of PCl5 is highlighted by its reaction with styrene to give, after hydrolysis, phosphonic acid derivatives.
Comparison with related reagents
Both PCl3 and PCl5 convert R3COH groups to the chloride R3CCl. The pentachloride is however a source of chlorine in many reactions. It chlorinates allylic andbenzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
CH bonds. PCl5 bears a greater resemblance to SO2Cl2, also a source of Cl2. For oxidative chlorinations on the laboratory scale, sulfuryl chloride is often preferred over PCl5 since the gaseous SO2 by-product is readily separated.
Chlorination of inorganic compounds
As for the reactions with organic compounds, the use of PCl5 has been superseded by SO2Cl2. The reaction of phosphorus pentoxide and PCl5 produces POCl3 : :6 PCl5 + P4O10 → 10 POCl3 PCl5 chlorinates nitrogen dioxide to form unstablenitryl chloride
Nitryl chloride is a volatile inorganic compound with formula ClNO2. At standard conditions it is a gas.
Formation
Nitryl chloride can be formed in the reaction of dinitrogen pentoxide with chlorides or hydrogen chloride:
:N2O5 + 2HCl → 2ClNO2 ...
:
:PCl5 + 2 NO2 → PCl3 + 2 NO2Cl
:2 NO2Cl → 2 NO2 + Cl2
PCl5 is a precursor for lithium hexafluorophosphate
Lithium hexafluorophosphate is an inorganic compound with the formula Li PF6. It is a white crystalline powder.
Production
LiPF6 is manufactured by reacting phosphorus pentachloride with hydrogen fluoride and lithium fluoride
:PCl5 + LiF + 5 ...
, LiPF6. Lithium hexafluorophosphate is a commonly employed salt in electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s in lithium ion batteries. is produced by the reaction of with lithium fluoride, with lithium chloride as a side product:
:PCl5 + 6 LiF → LiPF6 + 5 LiCl
Safety
PCl5 is a dangerous substance as it reacts violently with water. It is also corrosive when in contact with skin and can be fatal when inhaled.History
Phosphorus pentachloride was first prepared in 1808 by the English chemist Humphry Davy. Davy's analysis of phosphorus pentachloride was inaccurate; the first accurate analysis was provided in 1816 by the French chemist Pierre Louis Dulong. On p. 148, Dulong presented the correct analysis of phosphorus pentachloride (which is 14.9% phosphorus and 85.1% chlorine by weight, vs. Dulong's values of 15.4% and 84.6%, respectively).See also
*Phosphorus halides
In chemistry, there are three series of binary phosphorus halides, containing phosphorus in the oxidation states +5, +3 and +2. All compounds have been described, in varying degrees of detail, although serious doubts have been cast on the existenc ...
* Phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic a ...
* Phosphoryl chloride
* Phosphorus trifluorodichloride
Phosphorus trifluorodichloride is a chemical compound with the chemical formula PF3Cl2. The covalent molecule trigonal bipyramidal molecular geometry. The central phosphorus atom has sp3d hybridization, and the molecule has an asymmetric charge d ...
References
External links
The period 3 chlorides
{{Authority control Phosphorus chlorides Hypervalent molecules Phosphorus(V) compounds