|
Niobium Pentachloride
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation. Structure and properties Niobium(V) chloride forms chloro-bridged dimers in the solid state (''see'' figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond lengths are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction. NbBr5, TaCl5 and TaBr5 are isostructural with NbCl5, but NbI5 and TaI5 have different structures. Preparation Industrial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chloride. It is however possible to make generalizations when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known. Production and synthesis Industrial production Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina. : The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
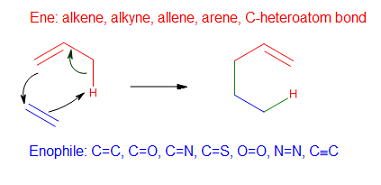
Carbonyl-ene Reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position. This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Many useful Lewis acid-catalyzed ene reactions have been also developed, which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products. Ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoniobium Compound
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common. Compound classes Carbonyls Unlike vanadium, which forms the neutral hexacarbonyl, niobium does not easily form an analogous complex. The salts of the anionic binary carbonyl, , are however well characterized. They are obtained by reduction of under an atmosphere of CO. Alkyl A wide variety of alkyl Nb compounds have been prepared. Low coordination number complexes require the absence of any β-hydrogen to prevent rapid β-hydride elimination. The simplest compounds are salts of , which is prepared by alkylation of using methyl lithium: : Cyclopentadienyl derivatives The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sol–gel Process
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution ('' sol'') that acts as the precursor for an integrated network (or ''gel'') of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol-gel process is used to produce ceramic nanoparticles. Stages In this chemical procedure, a " sol" (a colloidal solution) is formed that then gradually evolves towards the formation of a gel-like diphasic system containing both a liquid phase and solid phase whose morphologies range from discrete particles to continuous polymer networks. In the case of the colloid, the volume fraction of particles (or particle density) may be so low that a significant amount of fluid may need to be removed initially for the gel-like properti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium Pentoxide
Niobium pentoxide is the inorganic compound with the formula Nb2 O5. A colorless, insoluble, and fairly unreactive solid, it is the most widespread precursor for other compounds and materials containing niobium. It is predominantly used in alloying, with other specialized applications in capacitors, optical glasses, and the production of lithium niobate.Francois Cardarelli (2008) ''Materials Handbook'' Springer London Structure It has many polymorphic forms all based largely on octahedrally coordinated niobium atoms.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications The polymorphs are identified with a variety of prefixes. The form most commonly encountered is monoclinic H- Nb2 O5, which has a complex structure with a unit cell containing 28 niobium atoms and 70 oxygen, where 27 of the niobium atoms are octahedrally coordinated and one tetrahedrally. There is an uncharacterised solid hydrate, , the so-called niobic acid (previously calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride: This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled flask of sulfur dichloride. :SO3 + SCl2 -> SOCl2 + SO2 Other methods includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |