Particle aggregation on:
[Wikipedia]
[Google]
[Amazon]
Particle agglomeration refers to the formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of  Particle agglomeration can be a reversible or irreversible process. Particle agglomerates defined as "hard agglomerates" are more difficult to redisperse to the initial single particles. In the course of agglomeration, the agglomerates will grow in size, and as a consequence they may settle to the bottom of the container, which is referred to as
Particle agglomeration can be a reversible or irreversible process. Particle agglomerates defined as "hard agglomerates" are more difficult to redisperse to the initial single particles. In the course of agglomeration, the agglomerates will grow in size, and as a consequence they may settle to the bottom of the container, which is referred to as
 Often, colloidal particles are suspended in water. In this case, they accumulate a
Often, colloidal particles are suspended in water. In this case, they accumulate a
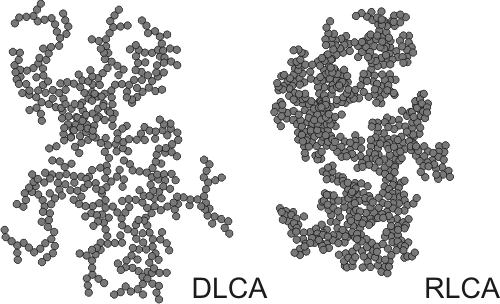 As the aggregation process continues, larger clusters form. The growth occurs mainly through encounters between different clusters, and therefore one refers to cluster-cluster aggregation process. The resulting clusters are irregular, but statistically self-similar. They are examples of mass
As the aggregation process continues, larger clusters form. The growth occurs mainly through encounters between different clusters, and therefore one refers to cluster-cluster aggregation process. The resulting clusters are irregular, but statistically self-similar. They are examples of mass
in Microgravity
{{DEFAULTSORT:Particle Aggregation Chemistry Materials science Colloidal chemistry
colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others exte ...
al systems. During this process, particles dispersed in the liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a thrombus, blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of co ...
or flocculation
In colloidal chemistry, flocculation is a process by which colloidal particles come out of Suspension (chemistry), suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The actio ...
and such a suspension is also called ''unstable''. Particle agglomeration can be induced by adding salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
or other chemicals referred to as ''coagulant'' or ''flocculant''.M. Elimelech, J. Gregory, X. Jia, R. Williams, ''Particle Deposition and Aggregation: Measurement, Modelling and Simulation'', Butterworth-Heinemann, 1998.
 Particle agglomeration can be a reversible or irreversible process. Particle agglomerates defined as "hard agglomerates" are more difficult to redisperse to the initial single particles. In the course of agglomeration, the agglomerates will grow in size, and as a consequence they may settle to the bottom of the container, which is referred to as
Particle agglomeration can be a reversible or irreversible process. Particle agglomerates defined as "hard agglomerates" are more difficult to redisperse to the initial single particles. In the course of agglomeration, the agglomerates will grow in size, and as a consequence they may settle to the bottom of the container, which is referred to as sedimentation
Sedimentation is the deposition of sediments. It takes place when particles in suspension settle out of the fluid in which they are entrained and come to rest against a barrier. This is due to their motion through the fluid in response to th ...
. Alternatively, a colloidal gel may form in concentrated suspensions which changes its rheological properties. The reverse process whereby particle agglomerates are re-dispersed as individual particles, referred to as peptization, hardly occurs spontaneously, but may occur under stirring or shear.
Colloidal particles may also remain dispersed in liquids for long periods of time (days to years). This phenomenon is referred to as ''colloidal stability'' and such a suspension is said to be functionally ''stable''. Stable suspensions are often obtained at low salt concentrations or by addition of chemicals referred to as ''stabilizers'' or ''stabilizing agents''. The stability of particles, colloidal or otherwise, is most commonly evaluated in terms of zeta potential. This parameter provides a readily quantifiable measure of interparticle repulsion, which is the key inhibitor of particle aggregation.
Similar agglomeration processes occur in other dispersed systems too. In emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloi ...
s, they may also be coupled to droplet coalescence, and not only lead to sedimentation but also to creaming. In aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be generated from natural or Human impact on the environment, human causes. The term ''aerosol'' co ...
s, airborne particles may equally aggregate and form larger clusters (e.g., soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. Soot is considered a hazardous substance with carcinogenic properties. Most broadly, the term includes all the particulate matter produced b ...
).
Early stages
A well dispersed colloidal suspension consists of individual, separated particles and is stabilized by repulsive inter-particle forces. When the repulsive forces weaken or become attractive through the addition of a coagulant, particles start to aggregate. Initially, particle doublets A2 will form from singlets A1 according to the schemeW. B. Russel, D. A. Saville, W. R. Schowalter,''Colloidal Dispersions'', Cambridge University Press, 1989. In the early stage of the aggregation process, the suspension mainly contains individual particles. The rate of this phenomenon is characterized by the aggregation rate coefficient . Since doublet formation is a second order rate process, the units of this coefficients are m3s−1 since particle concentrations are expressed asparticle number
In thermodynamics, the particle number (symbol ) of a thermodynamic system is the number of constituent particles in that system. The particle number is a fundamental thermodynamic property which is conjugate to the chemical potential. Unlike m ...
per unit volume (m−3). Since absolute aggregation rates are difficult to measure, one often refers to the dimensionless
Dimensionless quantities, or quantities of dimension one, are quantities implicitly defined in a manner that prevents their aggregation into units of measurement. ISBN 978-92-822-2272-0. Typically expressed as ratios that align with another sy ...
stability ratio , defined as where is the aggregation rate coefficient in the fast regime, and the coefficient at the conditions of interest. The stability ratio is close to unity in the fast regime, increases in the slow regime, and becomes very large when the suspension is stable.
 Often, colloidal particles are suspended in water. In this case, they accumulate a
Often, colloidal particles are suspended in water. In this case, they accumulate a surface charge
A surface charge is an electric charge present on a two-dimensional surface. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge ...
and an electrical double layer
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
forms around each particle. The overlap between the diffuse layers of two approaching particles results in a repulsive double layer interaction potential, which leads to particle stabilization. When salt is added to the suspension, the electrical double layer repulsion is screened, and van der Waals attraction become dominant and induce fast aggregation. The figure on the right shows the typical dependence of the stability ratio versus the electrolyte concentration, whereby the regimes of slow and fast aggregation are indicated.
The table below summarizes the critical coagulation concentration (CCC) ranges for different net charge of the counter ion.
The charge is expressed in units of elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
. This dependence reflects the Schulze–Hardy rule, which states that the CCC varies as the inverse sixth power of the counter ion charge. The CCC also depends on the type of ion somewhat, even if they carry the same charge. This dependence may reflect different particle properties or different ion affinities to the particle surface. Since particles are frequently negatively charged, multivalent metal cations thus represent highly effective coagulants.
Adsorption of oppositely charged species (e.g., protons, specifically adsorbing ions, surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent",
coined in ...
s, or polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
s) may destabilize a particle suspension by charge neutralization or stabilize it by buildup of charge, leading to a fast aggregation near the charge neutralization point, and slow aggregation away from it.
Quantitative interpretation of colloidal stability was first formulated within the DLVO theory. This theory confirms the existence slow and fast aggregation regimes, even though in the slow regime the dependence on the salt concentration is often predicted to be much stronger than observed experimentally. The Schulze–Hardy rule can be derived from DLVO theory as well.
Other mechanisms of colloid stabilization are equally possible, particularly, involving polymers. Adsorbed or grafted polymers may form a protective layer around the particles, induce steric repulsive forces, and lead to steric stabilization at it is the case with polycarboxylate ether (PCE), the last generation of chemically tailored superplasticizer specifically designed to increase the workability of concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
while reducing its water content to improve its properties and durability. When polymers chains adsorb to particles loosely, a polymer chain may bridge two particles, and induce bridging forces. This situation is referred to as bridging flocculation.
When particle aggregation is solely driven by diffusion, one refers to ''perikinetic'' aggregation. Aggregation can be enhanced through shear stress
Shear stress (often denoted by , Greek alphabet, Greek: tau) is the component of stress (physics), stress coplanar with a material cross section. It arises from the shear force, the component of force vector parallel to the material cross secti ...
(e.g., stirring). The latter case is called ''orthokinetic'' aggregation.
Later stages
 As the aggregation process continues, larger clusters form. The growth occurs mainly through encounters between different clusters, and therefore one refers to cluster-cluster aggregation process. The resulting clusters are irregular, but statistically self-similar. They are examples of mass
As the aggregation process continues, larger clusters form. The growth occurs mainly through encounters between different clusters, and therefore one refers to cluster-cluster aggregation process. The resulting clusters are irregular, but statistically self-similar. They are examples of mass fractal
In mathematics, a fractal is a Shape, geometric shape containing detailed structure at arbitrarily small scales, usually having a fractal dimension strictly exceeding the topological dimension. Many fractals appear similar at various scale ...
s, whereby their mass ''M'' grows with their typical size characterized by the radius of gyration ''R''g as a power-law
:
where ''d'' is the mass fractal dimension
In mathematics, a fractal dimension is a term invoked in the science of geometry to provide a rational statistical index of complexity detail in a pattern. A fractal pattern changes with the Scaling (geometry), scale at which it is measured.
It ...
. Depending whether the aggregation is fast or slow, one refers to diffusion limited cluster aggregation (DLCA) or reaction limited cluster aggregation (RLCA). The clusters have different characteristics in each regime. DLCA clusters are loose and ramified (''d'' ≈ 1.8), while the RLCA clusters are more compact (''d'' ≈ 2.1). The cluster size distribution is also different in these two regimes. DLCA clusters are relatively monodisperse, while the size distribution of RLCA clusters is very broad.
The larger the cluster size, the faster their settling velocity. Therefore, aggregating particles sediment and this mechanism provides a way for separating them from suspension. At higher particle concentrations, the growing clusters may interlink, and form a particle gel. Such a gel is an elastic solid body, but differs from ordinary solids by having a very low elastic modulus
An elastic modulus (also known as modulus of elasticity (MOE)) is a quantity that describes an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it.
Definition
The elastic modu ...
.
Homoaggregation versus heteroaggregation
When aggregation occurs in a suspension composed of similar monodisperse colloidal particles, the process is called ''homoaggregation'' (or ''homocoagulation''). When aggregation occurs in a suspension composed of dissimilar colloidal particles, one refers to ''heteroaggregation'' (or ''heterocoagulation''). The simplest heteroaggregation process occurs when two types of monodisperse colloidal particles are mixed. In the early stages, three types of doublets may form: While the first two processes correspond to homoaggregation in pure suspensions containing particles A or B, the last reaction represents the actual heteroaggregation process. Each of these reactions is characterized by the respective aggregation coefficients , , and . For example, when particles A and B bear positive and negative charge, respectively, the homoaggregation rates may be slow, while the heteroaggregation rate is fast. In contrast to homoaggregation, the heteroaggregation rate accelerates with decreasing salt concentration. Clusters formed at later stages of such heteroaggregation processes are even more ramified that those obtained during DLCA (''d'' ≈ 1.4). An important special case of a heteroaggregation process is the deposition of particles on a substrate. Early stages of the process correspond to the attachment of individual particles to the substrate, which can be pictures as another, much larger particle. Later stages may reflect blocking of the substrate through repulsive interactions between the particles, while attractive interactions may lead to multilayer growth, and is also referred to as ripening. These phenomena are relevant in membrane or filterfouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling, organic) or a non-living substance (inorganic). Fouling is usually distinguished from other surfac ...
.
Experimental techniques
Numerous experimental techniques have been developed to study particle aggregation. Most frequently used are time-resolved optical techniques that are based ontransmittance
Electromagnetic radiation can be affected in several ways by the medium in which it propagates. It can be Scattering, scattered, Absorption (electromagnetic radiation), absorbed, and Fresnel equations, reflected and refracted at discontinui ...
or scattering
In physics, scattering is a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including particles and radiat ...
of light.
Light transmission. The variation of transmitted light through an aggregating suspension can be studied with a regular spectrophotometer in the visible region. As aggregation proceeds, the medium becomes more turbid, and its absorbance increases. The increase of the absorbance can be related to the aggregation rate constant ''k'' and the stability ratio can be estimated from such measurements. The advantage of this
technique is its simplicity.
Light scattering. These techniques are based on probing the scattered light from an aggregating suspension in a time-resolved fashion. Static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight ''Mw'' of a macromolecule like a polymer or a protein in solution. Measurement of the scattering ...
yields the change in the scattering intensity, while dynamic light scattering the variation in the apparent hydrodynamic radius. At early-stages of aggregation, the variation of each of these quantities is directly proportional to the aggregation rate constant
''k''.
At later stages, one can obtain information on the clusters formed (e.g., fractal dimension). Light scattering works well for a wide range of particle sizes. Multiple scattering effects may have to be considered, since scattering becomes increasingly important for larger particles or larger aggregates. Such effects can be neglected in weakly turbid suspensions. Aggregation processes in strongly scattering systems have been studied with transmittance
Electromagnetic radiation can be affected in several ways by the medium in which it propagates. It can be Scattering, scattered, Absorption (electromagnetic radiation), absorbed, and Fresnel equations, reflected and refracted at discontinui ...
, backscattering techniques or diffusing-wave spectroscopy.
Single particle counting. This technique offers excellent resolution, whereby clusters made out of tenths of particles can be resolved individually. The aggregating suspension is forced through a narrow capillary particle counter and the size of each aggregate is being analyzed by light scattering. From the scattering intensity, one can deduce the size of each aggregate, and construct a detailed aggregate size distribution. If the suspensions contain high amounts of salt, one could equally use a Coulter counter
A Coulter counter is an apparatus for counting and sizing particles suspended in electrolytes. The Coulter counter is the commercial term for the technique known as resistive pulse sensing or electrical zone sensing. The apparatus is based on t ...
. As time proceeds, the size distribution shifts towards larger aggregates, and from this variation aggregation and breakup rates involving different clusters can be deduced. The disadvantage of the technique is that the aggregates are forced through a narrow capillary under high shear, and the aggregates may disrupt under these conditions.
Indirect techniques. As many properties of colloidal suspensions depend on the state of aggregation of the suspended particles, various indirect techniques have been used to monitor particle aggregation too. While it can be difficult to obtain quantitative information on aggregation rates or cluster properties from such experiments, they can be most valuable for practical applications. Among these techniques settling tests are most relevant. When one inspects a series of test tubes with suspensions prepared at different concentration of the flocculant, stable suspensions often remain dispersed, while the unstable ones settle. Automated instruments based on light scattering/transmittance to monitor suspension settling have been developed, and they can be used to probe particle aggregation. One must realize, however, that these techniques may not always reflect the actual aggregation state of a suspension correctly. For example, larger primary particles may settle even in the absence of aggregation, or aggregates that have formed a colloidal gel will remain in suspension. Other indirect techniques capable to monitor the state of aggregation include, for example, filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filte ...
, rheology
Rheology (; ) is the study of the flow of matter, primarily in a fluid (liquid or gas) state but also as "soft solids" or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applie ...
, absorption of ultrasonic waves, or dielectric properties.
Relevance
Particle aggregation is a widespread phenomenon, which spontaneously occurs in nature but is also widely explored in manufacturing. Some examples include. Formation of river delta. When river water carrying suspended sediment particles reaches salty water, particle aggregation may be one of the factors responsible for river delta formation. Charged particles are stable in river's fresh water containing low levels of salt, but they become unstable in sea water containing high levels of salt. In the latter medium, the particles aggregate, the larger aggregates sediment, and thus create the river delta.Papermaking
Papermaking is the manufacture of paper and cardboard, which are used widely for printing, writing, and packaging, among many other purposes. Today almost all paper is Pulp and paper industry, made using industrial machinery, while handmade pape ...
. Retention aids are added to the pulp to accelerate paper formation. These aids are coagulating aids, which accelerate the aggregation between the cellulose fibers and filler particles. Frequently, cationic polyelectrolytes are being used for that purpose.
Water treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, ...
. Treatment of municipal waste water normally includes a phase where fine solid particles are removed. This separation is achieved by addition of a flocculating or coagulating agent, which induce the aggregation of the suspended solids. The aggregates are normally separated by sedimentation, leading to sewage sludge. Commonly used flocculating agents in water treatment include multivalent metal ions (e.g., Fe3+ or Al3+), polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
s, or both.
Cheese making. The key step in cheese production is the separation of the milk into solid curds and liquid whey. This separation is achieved by inducing the aggregation processes between casein micelles by acidifying the milk or adding rennet. The acidification neutralizes the carboxylate groups on the micelles and induces the aggregation.
See also
*Aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be generated from natural or Human impact on the environment, human causes. The term ''aerosol'' co ...
*Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others exte ...
* Clarifying agent
*Double layer forces Double layer forces occur between charged objects across liquids, typically water. This force acts over distances that are comparable to the Debye length, which is on the order of one to a few tenths of Nanometre, nanometers. The strength of these ...
* DLVO theory (stability of colloids)
*Electrical double layer
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
*Emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloi ...
*Flocculation
In colloidal chemistry, flocculation is a process by which colloidal particles come out of Suspension (chemistry), suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The actio ...
* Gel
*Nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
*Particle deposition
Particle deposition is the spontaneous attachment of particles to surfaces. The particles in question are normally colloid, colloidal particles, while the surfaces involved may be planar, curved, or may represent particles much larger in size than ...
* Peptization
*Reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
*Settling
Settling is the process by which particulates move towards the bottom of a liquid and form a sediment. Particles that experience a force, either due to gravity or due to Centrifuge, centrifugal motion will tend to move in a uniform manner in the ...
* Smoluchowski coagulation equation
* Sol-gel
*Surface charge
A surface charge is an electric charge present on a two-dimensional surface. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge ...
*Suspension (chemistry)
In chemistry, a suspension is a heterogeneous mixture of a fluid that contains solid particles sufficiently large for sedimentation. The particles may be visible to the naked eye, usually must be larger than one micrometer, and will eventua ...
References
External links
in Microgravity
{{DEFAULTSORT:Particle Aggregation Chemistry Materials science Colloidal chemistry