PLCγ on:
[Wikipedia]
[Google]
[Amazon]
Phosphoinositide phospholipase C (PLC, EC 3.1.4.11, triphosphoinositide phosphodiesterase, phosphoinositidase C, 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase, monophosphatidylinositol phosphodiesterase, phosphatidylinositol phospholipase C, PI-PLC, 1-phosphatidyl-D-''myo''-inositol-4,5-bisphosphate inositoltrisphosphohydrolase; systematic name 1-phosphatidyl-1D-''myo''-inositol-4,5-bisphosphate inositoltrisphosphohydrolase) is a family of eukaryotic intracellular enzymes that play an important role in signal transduction processes. These enzymes belong to a larger superfamily of
1D-''myo''-inositol 1,4,5-trisphosphate + diacylglycerol PLCs catalyze the reaction in two sequential steps. The first reaction is a
 Phospholipase C performs a catalytic mechanism, depleting PIP2 and generating inositol trisphosphate (IP3) and diacylglycerol (DAG).
Depletion of PIP2 inactivates numerous effector molecules in the plasma membrane, most notably PIP2 dependent channels and transporters responsible for setting the cell's membrane potential.
The hydrolytic products also go on to modulate the activity of downstream proteins important for cellular signaling. IP3 is soluble, and diffuses through the cytoplasm and interacts with IP3 receptors on the
Phospholipase C performs a catalytic mechanism, depleting PIP2 and generating inositol trisphosphate (IP3) and diacylglycerol (DAG).
Depletion of PIP2 inactivates numerous effector molecules in the plasma membrane, most notably PIP2 dependent channels and transporters responsible for setting the cell's membrane potential.
The hydrolytic products also go on to modulate the activity of downstream proteins important for cellular signaling. IP3 is soluble, and diffuses through the cytoplasm and interacts with IP3 receptors on the
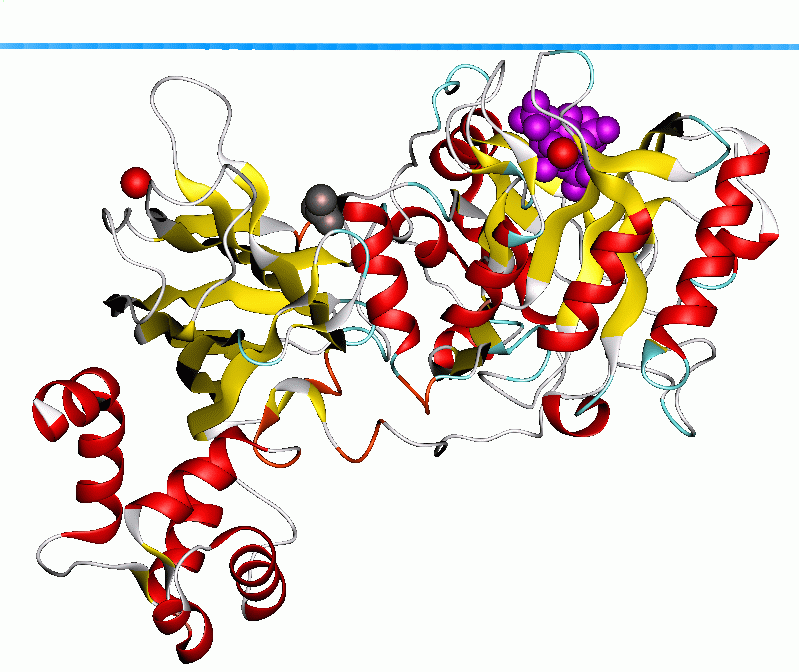 PLC-δ1 also possesses a classical leucine-rich
PLC-δ1 also possesses a classical leucine-rich
Phospholipase C
Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role ...
. Other families of phospholipase C enzymes have been identified in bacteria and trypanosomes. Phospholipases C are phosphodiesterases
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, ''phosphodiesterase'' refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many oth ...
.
Phospholipase Cs participate in phosphatidylinositol 4,5-bisphosphate (PIP2) metabolism and lipid signaling
Lipid signaling, broadly defined, refers to any biological signaling event involving a lipid messenger that binds a protein target, such as a receptor, kinase or phosphatase, which in turn mediate the effects of these lipids on specific cellular r ...
pathways in a calcium-dependent manner. At present, the family consists of six sub-families comprising a total of 13 separate isoforms that differ in their mode of activation, expression levels, catalytic regulation, cellular localization, membrane binding avidity and tissue distribution. All are capable of catalyzing the hydrolysis of PIP2 into two important second messenger
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. (Intercellular signals, a non-local form or cell signaling, encompassing both first me ...
molecules, which go on to alter cell responses such as proliferation
Proliferation may refer to:
Weapons
*Nuclear proliferation, the spread of nuclear weapons, material, and technology
*Chemical weapon proliferation, the spread of chemical weapons, material, and technology
* Small arms proliferation, the spread of ...
, differentiation, apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
, cytoskeleton remodeling, vesicular trafficking, ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of io ...
conductance, endocrine
The endocrine system is a messenger system comprising feedback loops of the hormones released by internal glands of an organism directly into the circulatory system, regulating distant target organs. In vertebrates, the hypothalamus is the neu ...
function and neurotransmission.
Reaction and catalytic mechanism
All family members are capable of catalyzing the hydrolysis of PIP2, a phosphatidylinositol at the inner leaflet of theplasma membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (t ...
into the two second messengers, inositol trisphosphate (IP3) and diacylglycerol (DAG).
The chemical reaction may be expressed as:
:1-phosphatidyl-1D-''myo''-inositol 4,5-bisphosphate + H2O 1D-''myo''-inositol 1,4,5-trisphosphate + diacylglycerol PLCs catalyze the reaction in two sequential steps. The first reaction is a
phosphotransferase
Phosphotransferases are a category of enzymes ( EC number 2.7) that catalyze phosphorylation reactions. The general form of the reactions they catalyze is:
:A-P + B \rightleftharpoons B-P + A
Where ''P'' is a phosphate group and A and B are the do ...
step that involves an intramolecular attack between the hydroxyl group at the 2' position on the inositol
Inositol, or more precisely ''myo''-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and ...
ring and the adjacent phosphate group resulting in a cyclic IP3 intermediate. At this point, DAG is generated. However, in the second phosphodiesterase step, the cyclic intermediate is held within the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
long enough to be attacked by a molecule of water, resulting in a final acyclic IP3 product. It should be mentioned that bacterial forms of the enzyme, which contain only the catalytic lipase
Lipase ( ) is a family of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually tr ...
domain, produce cyclic intermediates exclusively, whereas the mammalian isoforms generate predominantly the acyclic product. However, it is possible to alter experimental conditions (e.g., temperature, pH) ''in vitro'' such that some mammalian isoforms will alter the degree to which they produce mixtures of cyclic/acyclic products along with DAG. This catalytic process is tightly regulated by reversible phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
of different phosphoinositide
Phosphatidylinositol (or Inositol Phospholipid) consists of a family of lipids as illustrated on the right, where red is x, blue is y, and black is z, in the context of independent variation, a class of the phosphatidylglycerides. In such molecul ...
s and their affinity for different regulatory proteins.
Cell location
Phosphoinositide phospholipase C performs its catalytic function at theplasma membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (t ...
where the substrate PIP2 is present. This membrane docking is mediated mostly by lipid-binding domains (e.g. PH domain and C2 domain) that display affinity for different phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
components of the plasma membrane. It is important to note that research has also discovered that, in addition to the plasma membrane, phosphoinositide phospholipase C also exists within other sub-cellular regions such as the cytoplasm and nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
of the cell. At present, it is unclear exactly what the definitive roles for these enzymes in these cellular compartments are, particularly the nucleus.
Function
 Phospholipase C performs a catalytic mechanism, depleting PIP2 and generating inositol trisphosphate (IP3) and diacylglycerol (DAG).
Depletion of PIP2 inactivates numerous effector molecules in the plasma membrane, most notably PIP2 dependent channels and transporters responsible for setting the cell's membrane potential.
The hydrolytic products also go on to modulate the activity of downstream proteins important for cellular signaling. IP3 is soluble, and diffuses through the cytoplasm and interacts with IP3 receptors on the
Phospholipase C performs a catalytic mechanism, depleting PIP2 and generating inositol trisphosphate (IP3) and diacylglycerol (DAG).
Depletion of PIP2 inactivates numerous effector molecules in the plasma membrane, most notably PIP2 dependent channels and transporters responsible for setting the cell's membrane potential.
The hydrolytic products also go on to modulate the activity of downstream proteins important for cellular signaling. IP3 is soluble, and diffuses through the cytoplasm and interacts with IP3 receptors on the endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
, causing the release of calcium and raising the level of intracellular calcium.
''Further reading: Function of calcium in humans''
DAG remains within the inner leaflet of the plasma membrane due to its hydrophobic character, where it recruits protein kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and t ...
(PKC), which becomes activated in conjunction with binding calcium ions. This results in a host of cellular responses through stimulation of calcium-sensitive proteins such as Calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the bind ...
.
''Further reading: Function of protein kinase C
Domain structure
In terms of domain organization, all family members possess homologous X and Y catalytic domains in the form of a distorted Triose Phosphate Isomerase (TIM) barrel with a highly disordered, charged, and flexible intervening linker region. Likewise, all isoforms possess four EF hand domains, and a single C2 domain that flank the X and Y catalytic core. An N-terminal PH domain is present in every family except for the sperm-specific ζ isoform. SH2 (phosphotyrosine binding) and SH3 (proline-rich-binding) domains are found only in the γ form (specifically within the linker region), and only the ε form contains both guanine nucleotide exchange factor (GEF) and RA (Ras
Ras or RAS may refer to:
Arts and media
* RAS Records Real Authentic Sound, a reggae record label
* Rundfunk Anstalt Südtirol, a south Tyrolese public broadcasting service
* Rás 1, an Icelandic radio station
* Rás 2, an Icelandic radio stati ...
Associating) domains. The β subfamily is distinguished from the others by the presence of a long C-terminal extension immediately downstream of the C2 domain, which is required for activation by Gαq subunits, and which plays a role in plasma membrane binding and nuclear localization.
Isoenzymes and activation
The phospholipase C family consists of 13 isoenzymes split between six subfamilies, PLC-δ (1,3 & 4), -β(1-4), -γ( 1,2), -ε, -ζ, and the recently discovered -η(1,2) isoform. Depending on the specific subfamily in question, activation can be highly variable. Activation by either Gαq or Gβγ G-protein subunits (making it part of a G protein-coupled receptorsignal transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellula ...
pathway) or by transmembrane receptors with intrinsic or associated tyrosine kinase activity has been reported. In addition, members of the Ras superfamily of small GTPases (namely the Ras and Rho subfamilies) have also been implicated. It should also be mentioned that all forms of phospholipase C require calcium for activation, many of them possessing multiple calcium contact sites in the catalytic region. The only isoform that is known to be inactive at basal intracellular calcium levels is the δ subfamily of enzymes suggesting that they function as calcium amplifiers that become activated downstream of other PLC family members.
PLC-β
PLC-β(1-4) (120-155kDa) are activated by Gαq subunits through their C2 domain and long C-terminal extension. Gβγ subunits are known to activate the β2 and β3 isozymes only; however, this occurs through the PH domain and/or through interactions with the catalytic domain. The exact mechanism still requires further investigation. The PH domain of β2 and β3 plays a dual role, much like PLC-δ1, by binding to the plasma membrane, as well as being a site of interaction for the catalytic activator. However, PLC-β binds to the lipid surface independent of PIP2 with all isozymes preferring phosphoinositol-3-phosphate or neutral membranes. Members of the Rho GTPase family (e.g., Rac1, Rac2, Rac3, and cdc42) have been implicated in their activation by binding to an alternate site on the N-terminal PH domain followed by subsequent recruitment to theplasma membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (t ...
. A crystal structure of Rac1 bound to the PH domain of PLCβ2 has been solved. Like PLC-δ1, many PLC-β isoforms (in particular, PLC-β1) have been found to take up residence in the nuclear compartment. A basic amino acid region within the enzyme's long C-terminal tail appears to function as a Nuclear Localization Signal for import into the nucleus. PLC-β1 seems to play unspecified roles in cellular proliferation and differentiation.
PLC-γ
PLC-γ (120-155kDa) is activated by receptor and non-receptor tyrosine kinases due to the presence of two SH2 and a single SH3 domain situated between a split PH domain within the linker region. Although this particular isoform does not contain classic nuclear export or localization sequences, it has been found within the nucleus of certain cell lines. There are two main isoforms of PLCγ expressed in human specimens, PLC-γ1 and PLC-γ2.PLC-''Y2''
PLC-γ2 plays a major role in BCRsignal transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellula ...
. Absence of this enzyme in knockout specimens severely inhibits the development of B cells because the same signaling pathways necessary for antigen mediated B cell activation are necessary for B cell development from CLPs.
In B cell signaling, PI 3-kinase
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which i ...
is recruited to the BCR early in the signal transduction pathway. PI-3K phosphorylates PIP2 ( Phosphatidylinositol 4,5-bisphosphate) into PIP3 (Phosphatidylinositol 3,4,5-trisphosphate
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)''P''3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid t ...
). The increase in concentration of PIP3 recruits PLC-γ2 to the BCR complex which binds to BLNK
The B-cell linker protein is encoded by the ''BLNK'' gene and is an adaptor protein also known as SLP-65, BASH, and BCA. BLNK is expressed in B cells and macrophages and plays a large role in B cell receptor signalling, in a fashion analogous to ...
on the BCR scaffold and membrane PIP3. PLC-γ2 is then phosphorylated by Syk on one site and Btk on two sites. PLC-γ2 then competes with PI-3K for PIP2 which it hydrolyzes into IP3 (inositol 1,4,5-trisphosphate), which ultimately raises intercellular calcium, and diacylglycerol (DAG), which activates portions of the PKC family. Because PLC-γ2 competes for PIP2 with the original signaling molecule PI3K, it serves as a negative feedback
Negative feedback (or balancing feedback) occurs when some function (Mathematics), function of the output of a system, process, or mechanism is feedback, fed back in a manner that tends to reduce the fluctuations in the output, whether caused by ...
mechanism.
PLC-δ
The PLC-δ subfamily consists of three family members, δ1, 2, and 3. PLC-δ1 (85kDa) is the most well understood of the three. The enzyme is activated by high calcium levels generated by other PLC family members, and therefore functions as a calcium amplifier within the cell. Binding of its substrate PIP2 to the N-terminal PH domain is highly specific and functions to promote activation of the catalytic core. In addition, this specificity helps tether the enzyme tightly to the plasma membrane in order to access substrate through ionic interactions between the phosphate groups of PIP2 and charged residues in the PH domain. While the catalytic core does possess a weak affinity for PIP2, the C2 domain has been shown to mediate calcium-dependent phospholipid binding as well. In this model, the PH and C2 domains operate in concert as a "tether and fix" apparatus necessary for processive catalysis by the enzyme. PLC-δ1 also possesses a classical leucine-rich
PLC-δ1 also possesses a classical leucine-rich nuclear export signal
A nuclear export signal (NES) is a short target peptide containing 4 hydrophobic residues in a protein that targets it for export from the cell nucleus to the cytoplasm through the nuclear pore complex using nuclear transport. It has the opposite ...
(NES) in its EF hand motif, as well as a Nuclear localization signal within its linker region. These two elements combined allow PLC-δ1 to actively translocate into and out of the nucleus. However, its function in the nucleus remains unclear.
The widely expressed PLC-δ1 isoform is the best-characterized phospholipase family member, as it was the first to have high-resolution X-ray crystal structures available for analysis. In terms of domain architecture, all of the enzymes are built upon a common PLC-δ backbone, wherein each family displays similarities, as well as obvious distinctions, that contribute to unique regulatory properties within the cell. Because it is the only family found expressed in lower eukaryotic organisms such as yeast and slime molds, it is considered the prototypical PLC isoform. The other family members more than likely evolved from PLC-δ as their domain architecture and mechanism of activation were expanded. Although a ''full'' crystal structure has not been obtained, high-resolution X-ray crystallography has yielded the molecular structure of the N-terminal PH domain complexed with its product IP3, as well as the remainder of the enzyme with the PH domain ablated. These structures have provided researchers with the necessary information to begin speculating about other family members such as PLCβ2.
Other PLC families
*PLC-ε (230-260kDa ) is activated byRas
Ras or RAS may refer to:
Arts and media
* RAS Records Real Authentic Sound, a reggae record label
* Rundfunk Anstalt Südtirol, a south Tyrolese public broadcasting service
* Rás 1, an Icelandic radio station
* Rás 2, an Icelandic radio stati ...
and Rho
Rho (uppercase Ρ, lowercase ρ or ; el, ρο or el, ρω, label=none) is the 17th letter of the Greek alphabet. In the system of Greek numerals it has a value of 100. It is derived from Phoenician letter res . Its uppercase form uses the sa ...
GTPases.
*PLC-ζ (75kDa) is thought to play an important role in vertebrate fertilization by producing intracellular calcium oscillations important for the start of embryonic development. However, the mechanism of activation still remains unclear. This isoform is also capable of entering the early-formed pronucleus after fertilization, which seems to coincide with the cessation of calcium mobilization. It, like PLC-δ1 and PLC-β, possesses nuclear export and localization sequences.
*PLC-η has been implicated in neuronal functioning.
Human proteins in this family
PLCB1; PLCB2;PLCB3
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-3 is an enzyme that in humans is encoded by the ''PLCB3'' gene.
The gene codes for the enzyme phospholipase C β3. The enzyme catalyzes the formation of inositol 1,4,5-trisphosphate an ...
; PLCB4
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-4 is an enzyme that in humans is encoded by the ''PLCB4'' gene.
Function
The protein encoded by this gene catalyzes the formation of inositol 1,4,5-trisphosphate and diacylglycerol ...
; PLCD1
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-1 is an enzyme that in humans is encoded by the ''PLCD1'' gene.
PLCd1 is essential to maintain homeostasis of the skin.
See also
Phospholipase C
Phospholipase C (PLC) is a class of ...
; PLCD3
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-3 is an enzyme that in humans is encoded by the ''PLCD3'' gene.
Function
This gene encodes a member of the phospholipase C family, which catalyze the hydrolysis of phosphatidylin ...
; PLCD4
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-4 is an enzyme that in humans is encoded by the ''PLCD4'' gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units o ...
; PLCE1
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase epsilon-1 (PLCE1) is an enzyme that in humans is encoded by the ''PLCE1'' gene. This gene encodes a phospholipase enzyme (PLCE1) that catalyzes the hydrolysis of phosphatidylinositol-4,5-bis ...
;
PLCG1; PLCG2; PLCH1; PLCH2 PLCH may stand for:
* Plch, a municipality in the Czech Republic
* The Public Library of Cincinnati and Hamilton County
Cincinnati and Hamilton County Public Library (CHPL) is a public library system in the United States. In addition t ...
; PLCL1; PLCL2 PLCL may refer to:
* Park Lane College Leeds, a former British further education college, now part of Leeds City College
* ''Parker Lewis Can't Lose
''Parker Lewis Can't Lose'' is an American teen sitcom that originally aired on Fox from Sept ...
; PLCZ1
See also
* Clostridium perfringens alpha toxin *Lipid signaling
Lipid signaling, broadly defined, refers to any biological signaling event involving a lipid messenger that binds a protein target, such as a receptor, kinase or phosphatase, which in turn mediate the effects of these lipids on specific cellular r ...
* PH domain, found in some phospholipases C
* Phospholipase
* Zinc-dependent phospholipase C, a different family of phospholipase C
Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role ...
References
* * * * {{Portal bar, Biology, border=no EC 3.1.4 Peripheral membrane proteins Enzymes of known structure Signal transduction Protein families Enzymes Calcium enzymes Hydrolases Calcium signaling Cell signaling G protein-coupled receptors Cell biology de:Phospholipase C es:Fosfolipasa C fr:Phospholipase C he:פוספוליפאז C ru:Фосфолипаза C