|
PLCE1
1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase epsilon-1 (PLCE1) is an enzyme that in humans is encoded by the ''PLCE1'' gene. This gene encodes a phospholipase enzyme (PLCE1) that catalyzes the hydrolysis of phosphatidylinositol-4,5-bisphosphate to generate two second messengers: inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). Mutations in this gene cause early-onset nephrotic syndrome and have been associated with respiratory chain deficiency with diffuse mesangial sclerosis. Structure ''PLCE1'' is located on the q arm of chromosome 10 in position 23.33 and has 39 exons. PLCE1, the protein encoded by this gene, is located on the Golgi apparatus, the cell membrane, and in the cytosol. It contains 3 turns, 15 beta strands, and 6 alpha helixes. PLCE1 contains a 260 amino acid Ras-GEF domain at p. 531-790, a 149 amino acid PI-PLC X-box domain at p. 1392-1540, a 117 amino acid PI-PLC Y-box domain at p. 1730 – 1846, a 101 amino acid C2 dom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C2 Domain
A C2 domain is a protein structural domain involved in targeting proteins to cell membranes. The typical version (PKC-C2) has a beta-sandwich composed of 8 β-strands that co-ordinates two or three calcium ions, which bind in a cavity formed by the first and final loops of the domain, on the membrane binding face. Many other C2 domain families don't have calcium binding activity. Coupling with other domains C2 domains are frequently found coupled to enzymatic domains; for example, the C2 domain in PTEN, brings the phosphatase domain into contact with the plasma membrane, where it can dephosphorylate its substrate, phosphatidylinositol (3,4,5)-trisphosphate (PIP3), without removing it from the membrane - which would be energetically very costly. PTEN consists of two domains, a protein tyrosine phosphatase domain and a C2 domain. This domain pair constitutes a superdomain, a heritable unit that is found in various proteins in fungi, plants and animals. In addition, phospha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RasGEF Domain
RasGEF domain is domain found in the CDC25 family of guanine nucleotide exchange factors for Ras-like small GTPases. Ras proteins are membrane-associated molecular switches that bind GTP and GDP and slowly hydrolyze GTP to GDP. The balance between the GTP bound (active) and GDP bound (inactive) states is regulated by the opposite action of proteins activating the GTPase activity and that of proteins which promote the loss of bound GDP and the uptake of fresh GTP. The latter proteins are known as guanine-nucleotide dissociation stimulators (GDSs) (or also as guanine-nucleotide releasing (or exchange) factors (GRFs)). Proteins that act as GDS can be classified into at least two families, on the basis of sequence similarities, the CDC24 family (see ) and this CDC25 (RasGEF) family. The size of the proteins of the CDC25 family range from 309 residues (LTE1) to 1596 residues (sos). The sequence similarity shared by all these proteins is limited to a region of about 250 amino acids g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RHOA
Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the ''RHOA'' gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 (Rho-associated, coiled-coil containing protein kinase 1) and DIAPH1 (Diaphanous Homologue 1, a.k.a. hDia1, homologue to mDia1 in mouse, diaphanous in ''Drosophila'') are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Focal Segmental Glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS) is a histopathologic finding of scarring (sclerosis) of glomeruli and damage to renal podocytes.Rosenberg, Avi Z.; Kopp, Jeffrey B. (2017-03-07). "Focal Segmental Glomerulosclerosis". ''Clinical Journal of the American Society of Nephrology''. 12 (3): 502–517. doi:10.2215/CJN.05960616. ISSN 1555-9041. PMC 5338705. PMID 28242845.D'Agati V. The many masks of focal segmental glomerulosclerosis. Kidney Int. 1994 Oct;46(4):1223-41. doi: 10.1038/ki.1994.388. . This process damages the filtration function of the kidney, resulting in protein loss in the urine. FSGS is a leading cause of excess protein loss— nephrotic syndrome—in children and adults.Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004 Nov;44(5):815-25. . Signs and symptoms include proteinuria, water retention, and edema.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM. Focal segment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edema
Edema, also spelled oedema, and also known as fluid retention, dropsy, hydropsy and swelling, is the build-up of fluid in the body's tissue. Most commonly, the legs or arms are affected. Symptoms may include skin which feels tight, the area may feel heavy, and joint stiffness. Other symptoms depend on the underlying cause. Causes may include venous insufficiency, heart failure, kidney problems, low protein levels, liver problems, deep vein thrombosis, infections, angioedema, certain medications, and lymphedema. It may also occur after prolonged sitting or standing and during menstruation or pregnancy. The condition is more concerning if it starts suddenly, or pain or shortness of breath is present. Treatment depends on the underlying cause. If the underlying mechanism involves sodium retention, decreased salt intake and a diuretic may be used. Elevating the legs and support stockings may be useful for edema of the legs. Older people are more commonly affected. The wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinuria
Proteinuria is the presence of excess proteins in the urine. In healthy persons, urine contains very little protein; an excess is suggestive of illness. Excess protein in the urine often causes the urine to become foamy (although this symptom may also be caused by other conditions). Severe proteinuria can cause nephrotic syndrome in which there is worsening swelling of the body. Signs and symptoms Proteinuria often causes no symptoms and it may only be discovered incidentally. Foamy urine is considered a cardinal sign of proteinuria, but only a third of people with foamy urine have proteinuria as the underlying cause. It may also be caused by bilirubin in the urine ( bilirubinuria), retrograde ejaculation, pneumaturia (air bubbles in the urine) due to a fistula, or drugs such as pyridium. Causes There are three main mechanisms to cause proteinuria: * Due to disease in the glomerulus * Because of increased quantity of proteins in serum (overflow proteinuria) * Due to low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. These products are often proteins, but in non-protein-coding genes such as transfer RNA (tRNA) and small nuclear RNA (snRNA), the product is a functional non-coding RNA. Gene expression is summarized in the central dogma of molecular biology first formulated by Francis Crick in 1958, further developed in his 1970 article, and expanded by the subsequent discoveries of reverse transcription and RNA replication. The process of gene expression is used by all known life— eukaryotes (including multicellular organisms), prokaryotes (bacteria and archaea), and utilized by viruses—to generate the macromolecular machinery for life. In genetics, gene expression is the most fundamental level at which the genotype gives rise to the phen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inositol-trisphosphate 3-kinase
Inositol (1,4,5) trisphosphate 3-kinase (), abbreviated here as ITP3K, is an enzyme that facilitates a phospho-group transfer from adenosine triphosphate to 1D-myo-inositol 1,4,5-trisphosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups ( phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. ITP3K catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate. ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position, producing Ins(1,3,4,5)P4, also known as inositol tetrakisphosphate or IP4. In biology, the enzyme ITP3K is abbreviated a number of different ways, including 1D-myo-inositol-trisphosphate 3-kinase, ITP3K, ITPK, IP3-kinase, IP3-3-kinase, Ins(1,4,5)P3 3-kinase. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylinositol-4,5-bisphosphate
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)''P''2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)''P''2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins. PIP2 is formed primarily by the type I phosphatidylinositol 4-phosphate 5-kinases from PI(4)P. In metazoans, PIP2 can also be formed by type II phosphatidylinositol 5-phosphate 4-kinases from PI(5)P. The fatty acids of PIP2 are variable in different species and tissues, but the most common fatty acids are stearic in position 1 and arachidonic in 2. Signaling pathways PIP2 is a part of many cellular signaling pathways, including PIP2 cycle, PI3K signalling, and PI5P metabolism. Recently, it has been found in the nucleus with unknown function. Functions Cytoskeleton dynamics near membranes PIP2 regulates the organization, polymerization, and bra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipase
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze: *Phospholipase A ** Phospholipase A1 – cleaves the ''sn''-1 acyl chain (where ''sn'' refers to stereospecific numbering). **Phospholipase A2 – cleaves the ''sn''-2 acyl chain, releasing arachidonic acid. *Phospholipase B – cleaves both ''sn''-1 and ''sn''-2 acyl chains; this enzyme is also known as a lysophospholipase. *Phospholipase C – cleaves before the phosphate, releasing diacylglycerol and a phosphate-containing head group. PLCs play a central role in signal transduction, releasing the second messenger inositol triphosphate. * Phospholipase D – ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
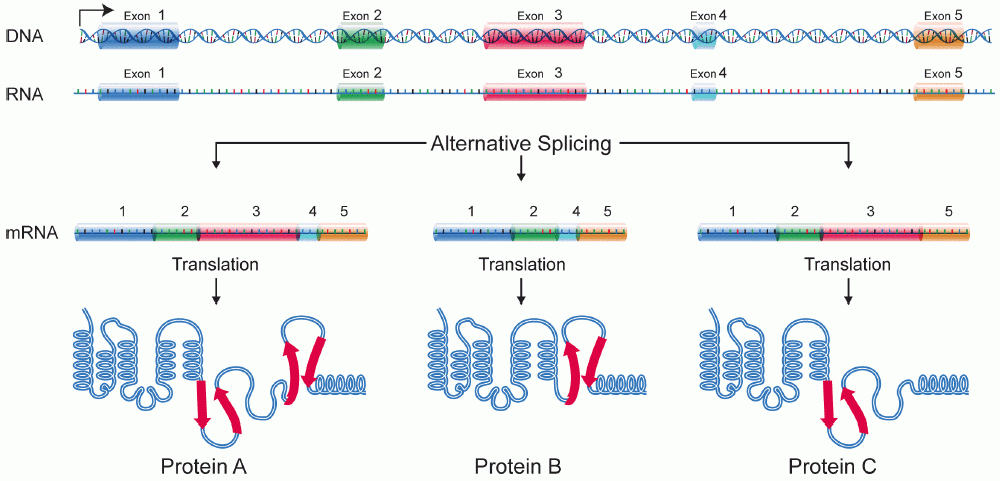
Protein Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments (exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions genes revealed by the human genome project and the large diversity of proteins seen in an organism: differen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ca²⁺
Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation. Plasma calcium levels in mammals are tightly regulated, electronic-book electronic- with bone acting as the major mineral storage site. Calcium ions, Ca2+, are released from bone into the bloodstream under controlled conditions. Calcium is transported through the bloodstream as dissolved ions or bound to proteins such as serum albumin. Parathyroid hormone secreted by the parathyroid gland regulates the resorption of Ca2+ from bone, reabsor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |