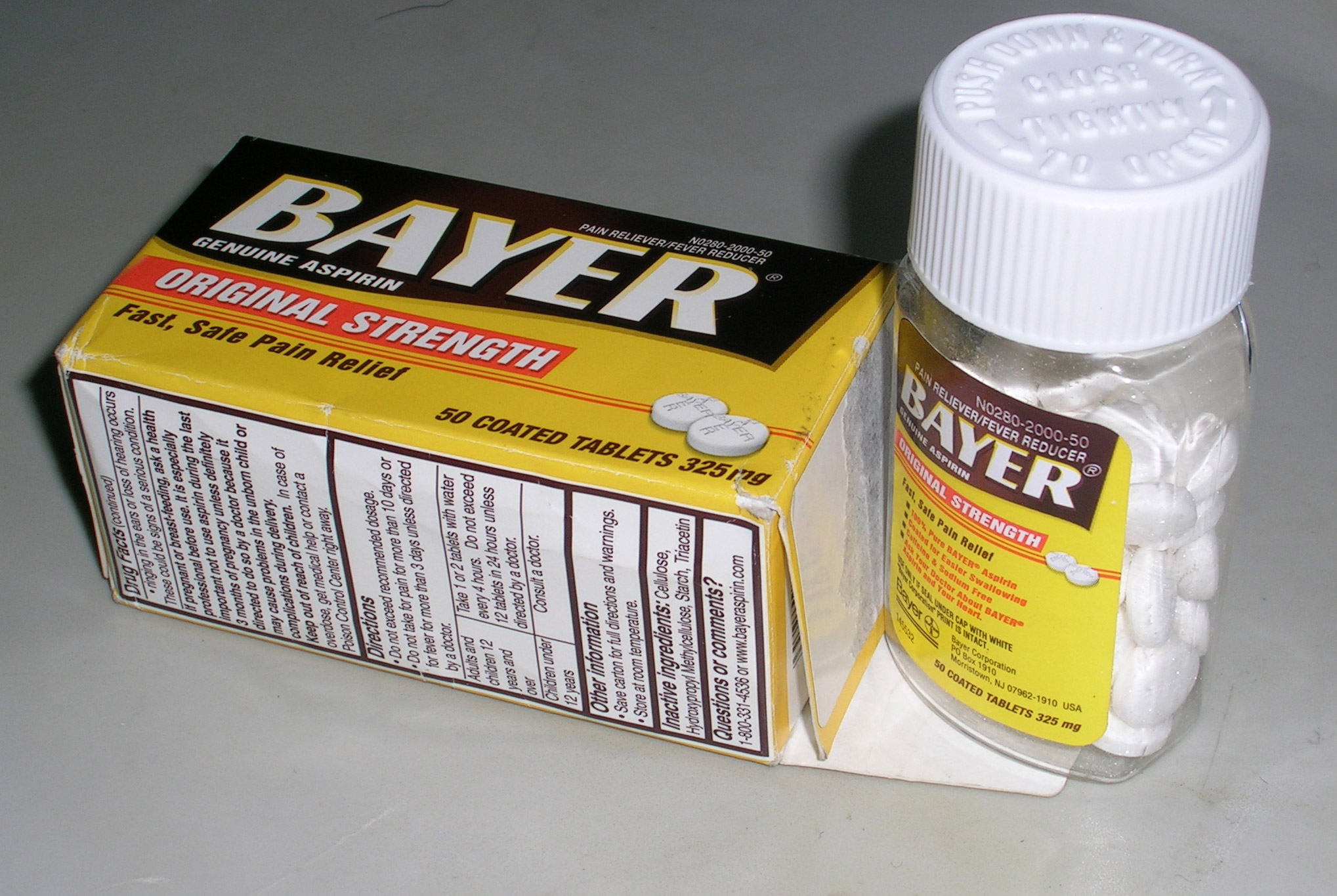
 Over-the-counter (OTC) drugs are
Over-the-counter (OTC) drugs are medicine
Medicine is the science and Praxis (process), practice of caring for a patient, managing the diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, and Health promotion ...
s sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs
A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The rea ...
, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency
A regulatory agency (regulatory body, regulator) or independent agency (independent regulatory agency) is a government authority that is responsible for exercising autonomous dominion over some area of human activity in a licensing and regulat ...
to ensure that they contain ingredients that are safe and effective when used without a physician
A physician (American English), medical practitioner (Commonwealth English), medical doctor, or simply doctor, is a health professional who practices medicine, which is concerned with promoting, maintaining or restoring health through th ...
's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) rather than final products. By regulating APIs instead of specific drug formulations, governments allow manufacturers the freedom to formulate ingredients, or combinations of ingredients, into proprietary mixtures.
The term ''over-the-counter'' (''OTC'') refers to a medication that can be purchased without a medical prescription. In contrast, prescription drug
A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The rea ...
s require a prescription from a doctor or other health care professional and should only be used by the prescribed individual. Some drugs may be legally classified as over-the-counter (i.e. no prescription is required), but may only be dispensed by a pharmacist
A pharmacist, also known as a chemist (Commonwealth English) or a druggist (North American and, archaically, Commonwealth English), is a healthcare professional who prepares, controls and distributes medicines and provides advice and instructi ...
after an assessment of the patient's needs or the provision of patient education. Regulations detailing the establishments where drugs may be sold, who is authorized to dispense them, and whether a prescription is required vary considerably from country to country.
Usage
As of 2011, around a third of older adults in the U.S. reportedly used OTC drugs. By 2018, the prevalence of use by adults in the U.S. as first-line treatment for minor illnesses had reached 81%.Regulation by country
Canada
InCanada
Canada is a country in North America. Its ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, covering over , making it the world's second-largest country by tota ...
, there are four drug schedules:
* Schedule 1: Requires a prescription for sale and is provided to the public by a licensed pharmacist.
* Schedule 2: Does not require a prescription but requires an assessment by a pharmacist prior to sale. These drugs are kept in an area of the pharmacy where there is no public access and may also be referred to as "behind-the-counter" drugs.
* Schedule 3: Does not require a prescription but must be kept in an area under the supervision of a pharmacist. These drugs are kept in an area of the retail outlet where self-selection is possible, but a pharmacist must be available to assist in the self-selection of medication if required.
* Unscheduled: Does not require a prescription and may be sold in any retail outlet.
All medications other than Schedule 1 may be considered an OTC drug, as they do not require prescriptions for sale. While thNational Association of Pharmacy Regulatory Authorities
provides recommendations on the scheduling of drugs for sale in Canada, each province may determine its own scheduling. The drugs found in each schedule may vary from province to province.
India
In November 2016, India's Drug Consultative Committee announced it was embarking on establishing a definition of drugs which could be dispensed without a prescription. Prior to this, the general assumption was that any drug which did not fall into a prescription schedule could be purchased without a prescription. However, the needed definition had not been enacted by early 2018. The lack of a legal definition for OTC drugs has led to this market segment being effectively unregulated.Netherlands
In theNetherlands
)
, anthem = ( en, "William of Nassau")
, image_map =
, map_caption =
, subdivision_type = Sovereign state
, subdivision_name = Kingdom of the Netherlands
, established_title = Before independence
, established_date = Spanish Netherl ...
, there are four categories:
* UR (Uitsluitend Recept): prescription only
* UA (Uitsluitend Apotheek): pharmacist only
* UAD (Uitsluitend Apotheek of Drogist): pharmacist or drugstore only
* AV (Algemene Verkoop): may be sold in general stores
A drug that is UA may be sold OTC but only by pharmacists. The drug can be on the shelves like any other product. Examples are domperidone
Domperidone, sold under the brand name Motilium among others, is a dopamine antagonist medication which is used to treat nausea and vomiting and certain gastrointestinal problems like gastroparesis (delayed gastric emptying). It raises th ...
, 400 mg ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus ...
up to 50 tablets and dextromethorphan
Dextromethorphan (DXM) is a medication most often used as a cough suppressant in over-the-counter cold and cough medicines. It is sold in syrup, tablet, spray, and lozenge forms. In 2022, the FDA approved a formulation of it combined with bu ...
. A drug that is UAD can also be sold at drugstores which are stores where no prescription can be filled. The drugs are usually on the shelves, and the store also sells items like toys, gadgets, perfumes and homeopathic products. The drugs in this category have limited risk and addiction potential. Examples are naproxen
Naproxen is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain, menstrual cramps, inflammatory diseases such as rheumatoid arthritis, gout and fever. It is taken orally. It is available in immediate and delayed release formula ...
and diclofenac
Diclofenac, sold under the brand name Voltaren, among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory diseases such as gout. It is taken by mouth or rectally in a suppository, used by injection, o ...
in small amounts, cinnarizine, 400 mg ibuprofen up to 20 tablets and also 500 mg paracetamol
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. Common brand names include Tylenol and Panadol.
At a standard dose, paracetamol only slightly decreases body temperature; it is inferi ...
up to 50 tablets. Drugs in the AV category can be sold at supermarkets
A supermarket is a self-service shop offering a wide variety of food, beverages and household products, organized into sections. This kind of store is larger and has a wider selection than earlier grocery stores, but is smaller and more limite ...
, gas stations, etc. and include only drugs with minimal risk to the public, like paracetamol up to 20 tablets, 200 mg ibuprofen up to 10 tablets, cetirizine
Cetirizine, sold under the brand name Zyrtec among others, is a second-generation antihistamine used to treat allergic rhinitis (hay fever), dermatitis, and urticaria (hives). It is taken by mouth. Effects generally begin within thirty minutes ...
and loperamide
Loperamide, sold under the brand name Imodium, among others,Drugs.co Page accessed September 4, 2015 is a medication used to decrease the frequency of diarrhea. It is often used for this purpose in inflammatory bowel disease and short bowel syn ...
.
United States
In theUnited States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., federal district, five ma ...
, the manufacture and sale of OTC substances are regulated by the Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
. The FDA requires that all "new drugs" obtain a New Drug Application (NDA) before entering interstate commerce, but the act exempts any drugs generally recognized as safe and effective (GRAS/E). To deal with the vast number of OTC drugs that were already on the market before the requirement that all drugs obtain an NDA, the FDA created the OTC monograph system to review classes of drugs and to categorize them as GRAS/E after review by expert panels. Certain classes of OTC drugs would not be required to obtain an NDA and could remain on the market if they conformed to the monograph guidelines for doses, labeling, and warnings finalized in the Code of Federal Regulations
In the law of the United States, the ''Code of Federal Regulations'' (''CFR'') is the codification of the general and permanent regulations promulgated by the executive departments and agencies of the federal government of the United States. ...
Thus, an OTC drug product is allowed to be marketed either (1) pursuant to an FDA monograph or (2) pursuant to an NDA for products that do not fit within a specific monograph. There is also the possibility that certain OTC drug products are marketed under the grandfathering provisions of the Federal Food, Drug, and Cosmetic Act
The United States Federal Food, Drug, and Cosmetic Act (abbreviated as FFDCA, FDCA, or FD&C) is a set of laws passed by the United States Congress in 1938 giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of f ...
, but the FDA has never formally acknowledged that any legitimate grandfathered OTC drug exists.
Examples of OTC substances approved in the United States are sunscreens, anti-microbial and anti-fungal products, external and internal analgesics such as lidocaine
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia. When used for local anaesthesia or in nerve blocks, li ...
and aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat in ...
, psoriasis
Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by raised areas of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small, localized patches to complet ...
and eczema
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can v ...
topical treatments, anti-dandruff
Dandruff is a skin condition that mainly affects the scalp. Symptoms include flaking and sometimes mild itchiness. It can result in social or self-esteem problems. A more severe form of the condition, which includes inflammation of the skin, ...
shampoos containing coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoria ...
, and other topical products with a therapeutic effect.
The Federal Trade Commission regulates advertising of OTC products, in contrast to prescription drug advertising, which is regulated by the FDA.
The FDA requires OTC products to be labeled with an approved "Drug Facts" label to educate consumers about their medications. The labels comply to a standard format and are intended to be easy for typical consumers to understand. Drug Facts labels include information on the product's active ingredient(s), indication
Indication may refer to:
* A synonym for sign
* Human interface, highlighting the single object pointed to as a cursor is moved, without any other user action such as clicking, is indication
* Indication (medicine). A valid reason to use a certain ...
s and purpose, safety warnings, directions for use, and inactive ingredients
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts (thus often referred ...
.
The 2020 Coronavirus Aid, Relief, and Economic Security Act
The Coronavirus Aid, Relief, and Economic Security Act, also known as the CARES Act, is a $2.2trillion Stimulus (economics), economic stimulus bill passed by the 116th U.S. Congress and signed into law by President Donald Trump on March 27, 20 ...
(CARES Act) includes reforms that modernize the way certain OTC drugs are regulated in the United States. Many OTC monographs need to be updated but updating or changing an OTC monograph requires the slow and burdensome notice-and-comment rulemaking process. The CARES Act includes OTC monograph reform provisions that replace the rulemaking process with an administrative order process.
Restricted over-the-counter substances
An ill-defined third category of substances is products having over-the-counter status from the FDA while being simultaneously subject to other restrictions on sale. While they are legally classified as OTC drugs, they are typically stored behind the counter and are sold only in stores that are registered with their state. They may be unavailable in convenience and grocery stores that stock other non-restricted OTC medications. For example, many drugstores have moved products containingpseudoephedrine
Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.
It was first charac ...
, an OTC product, into locations where customers must ask a pharmacist for them. A prescription is not required; the change has been made in an effort to reduce methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Meth ...
production. Since the passage of the Illinois Methamphetamine Precursor Control Act The Methamphetamine Precursor Control Act (MPCA, ) is an Illinois state law that was signed into law on November 16, 2005, and took effect on January 15, 2006. The MPCA is an act used to create significant barriers such as the requirement to presen ...
and the subsequent federal Combat Methamphetamine Epidemic Act of 2005
The Combat Methamphetamine Epidemic Act of 2005 (CMEA) is federal legislation enacted in the United States on March 9, 2006, to regulate, among other things, retail over-the-counter sales of following products because of their use in the manufact ...
, the purchase of pseudoephedrine is restricted. Sellers of pseudoephedrine must obtain and record the identity of the purchaser and enforce quantity restrictions. After initial attempts to control methamphetamine use (by requiring documentation of sale with government issued ID as well as limits on the quantity an individual could purchase) failed to realize meaningful reductions in methamphetamine use and production, Mississippi
Mississippi () is a state in the Southeastern region of the United States, bordered to the north by Tennessee; to the east by Alabama; to the south by the Gulf of Mexico; to the southwest by Louisiana; and to the northwest by Arkansas. Mis ...
passed House Bill 512 in the State Senate on February 2, 2010 "to require a prescription from a licensed medical professional to purchase over-the-counter medicines with pseudoephedrine, ephedrine
Ephedrine is a central nervous system (CNS) stimulant that is often used to prevent low blood pressure during anesthesia. It has also been used for asthma, narcolepsy, and obesity but is not the preferred treatment. It is of unclear benefit in n ...
, or any other precursor chemical that can readily and illicitly be converted into methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Meth ...
, Methcathinone
Methcathinone (α- methylamino- propiophenone or ephedrone) (sometimes called "cat" or "jeff" or "catnip" or "M-Kat" or "kat" or "intash" ) is a monoamine alkaloid and psychoactive stimulant, a substituted cathinone. It is used as a recreation ...
or any active/scheduled analogs of Phenylethylamines
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amin ...
/ amphetamine
Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used ...
." However, products containing the substance are still OTC in most states, since no prescription is required.
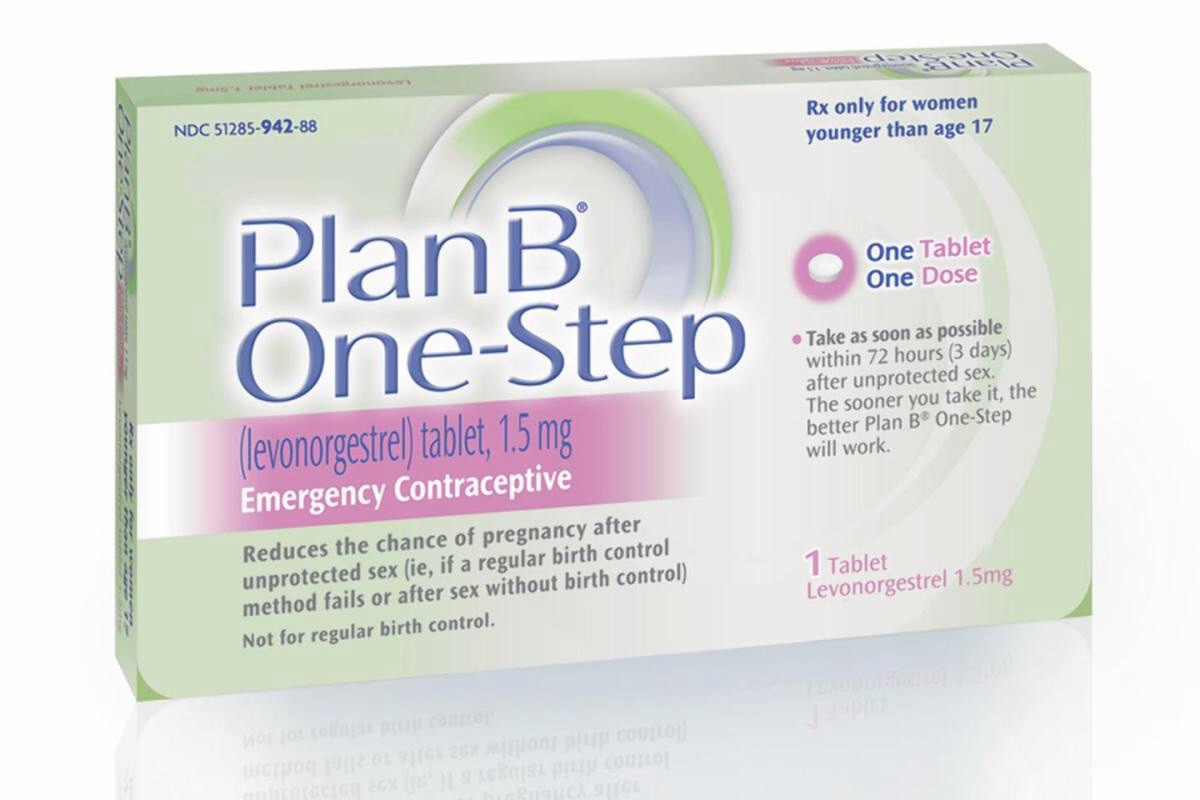 A similar regulation once applied to some forms of
A similar regulation once applied to some forms of emergency contraception
Emergency contraception (EC) is a birth control measure, used after sexual intercourse to prevent pregnancy.
There are different forms of EC. Emergency contraceptive pills (ECPs), sometimes simply referred to as emergency contraceptives (ECs), ...
. However, on February 25, 2014, the FDA approved generic one-pill emergency contraception products for unrestricted sale on the shelf. There is no age limit or need for ID to purchase.
Furthermore, some Schedule V controlled substances may be classified as OTC products in certain states. Such drugs are sold without a prescription but are subject to record-keeping rules and quantity and/or age restrictions, and they must be dispensed by a pharmacy
Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medication, medicines. It is a miscellaneous science as it ...
. Finally, pharmacies frequently require a prescription for Schedule V drugs as a matter of policy, despite their OTC status according to applicable laws and regulations.
United Kingdom
In the United Kingdom, medication is governed by the Medicines Regulations 2012. Medication falls into one of three categories: # Prescription Only Medication (POM), which are legally available only with a valid prescription from a prescriber. Apharmacist
A pharmacist, also known as a chemist (Commonwealth English) or a druggist (North American and, archaically, Commonwealth English), is a healthcare professional who prepares, controls and distributes medicines and provides advice and instructi ...
has to be on the premises for POM medicines to be dispensed, required by law. The medicine has been specifically prescribed for the patient holding the prescription, so it is considered safe for only the recipient to take. Just a small example of these include most antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention ...
and all antidepressants
Antidepressants are a class of medication used to treat major depressive disorder, anxiety disorders, chronic pain conditions, and to help manage addictions. Common side-effects of antidepressants include dry mouth, weight gain, dizziness, heada ...
or antidiabetic
Drugs used in diabetes treat diabetes mellitus by altering the glucose level in the blood. With the exceptions of insulin, most GLP receptor agonists (liraglutide, exenatide, and others), and pramlintide, all are administered orally and are thus ...
medications. Certain POM medicines are additionally marked Controlled Drug (CD) due to risk of abuse and the possibility of diversion for sale as street drugs. Examples of CDs include all benzodiazepines
Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, ...
and strong opioids such as heroin and fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
.
# General Sales List (GSL), available off the shelf with no pharmacy training required to sell (so they can be sold anywhere, such as supermarkets). In general, they are considered safe for most people when taken correctly. Examples of these include 16-packs (or less) of painkillers such as paracetamol
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. Common brand names include Tylenol and Panadol.
At a standard dose, paracetamol only slightly decreases body temperature; it is inferi ...
, ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus ...
, and aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat in ...
as well as a host of other medications such as small pack sizes of some antihistamine
Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic (not patented) drug that can be bought without a prescription and provide ...
s, some laxative
Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation.
Laxatives vary as to how they work and the side effects they may have. Certain stimulant, lub ...
medication, and skin creams. Also includes the recreational
Recreation is an activity of leisure, leisure being discretionary time. The "need to do something for recreation" is an essential element of human biology and psychology. Recreational activities are often done for enjoyment, amusement, or pleasu ...
substances alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
, caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
, and some nicotine preparations.
# Pharmacy Medicines (P) are medicines that are legally neither a POM or GSL medication. These can be sold from a registered pharmacy but should not be available for self-selection (although directions to discuss a 'P' product may be allocated shelf space with associated GSL items). 'P' medications are reserved from the GSL list as they are either associated with a need for advice on use, or used in conditions which may require referral to a medical prescriber. Suitable trained counter assistants may sell a 'P' medication under the supervision of a pharmacist and will ask questions to determine if the customer needs to be referred for a discussion with a pharmacist. Some 'POM' medicines are available for use in certain situations and doses as 'P' medicines.
If it is not appropriate to sell a 'P' medication – i.e. the condition is not suitable for self-management and requires referral to a medical prescriber – then a sale should not occur and the pharmacist has a legal and professional obligation to refer this on to an appropriate service.
Examples of these include some sleep aid tablets such as diphenhydramine
Diphenhydramine (DPH) is an antihistamine and sedative mainly used to treat allergies, insomnia, and symptoms of the common cold. It is also less commonly used for tremor in parkinsonism, and nausea. It is taken by mouth, injected into a v ...
, human deworming
Deworming (sometimes known as worming, drenching or dehelmintization) is the giving of an anthelmintic drug (a wormer, dewormer, or drench) to a human or animals to rid them of helminths parasites, such as roundworm, flukes and tapeworm. Pur ...
tablets such as Mebendazole
Mebendazole (MBZ), sold under the brand name Vermox among others, is a medication used to treat a number of parasitic worm infestations. This includes ascariasis, pinworm infection, hookworm infections, guinea worm infections, hydatid disea ...
, painkillers with small amounts of codeine
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, ''Papaver somniferum''. It is typically use ...
(up to 12.8 mg per tablet), and pseudoephedrine
Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.
It was first charac ...
. Medication available only with a prescription is marked somewhere on the box/container with OM Pharmacy-only products are marked with A prescription is not required for medicines, and pharmacy sales assistants are required by Royal Pharmaceutical Society codes to ask certain questions, which varies for what the customer says. If they ask for a specific product, the pharmacy assistant must ask "Who is it for," "How long have you had the symptoms," "Are you allergic to any medication," "Are you taking any medication" ('WHAM' questions). If a customer asks for a remedy, e.g., hay fever, then the two WHAM questions must be followed "Who is it for," "What are the symptoms," "How long have you had the symptoms," "Have you taken any action towards your symptoms," and "Are you taking any other medication." It is with this information that the pharmacist can halt the sale, if need be. No OM or SLproducts that are stocked in a pharmacy can be sold, dispensed, or pre-made until a responsible pharmacist is signed in and on the premises. Some medication available in supermarkets and petrol stations is sold only in smaller packet sizes. Often, larger packs will be marked as and available only from a pharmacy. Frequently, customers buying larger-than-usual doses of medicines (such as DXM
Dextromethorphan (DXM) is a medication most often used as a cough suppressant in over-the-counter cold and cough medicines. It is sold in syrup, tablet, spray, and lozenge forms. In 2022, the FDA approved a formulation of it combined with bupr ...
, promethazine, codeine or Gee's Linctus
Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including a ...
) will be queried, due to the possibility of abuse.
Transitions between prescription and OTC
As a general rule, over-the-counter drugs have to be used primarily to treat a condition that does not require the direct supervision of a doctor and must be proven to be reasonably safe and well tolerated. OTC drugs are usually also required to have little or no abuse potential, although in some areas drugs such ascodeine
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, ''Papaver somniferum''. It is typically use ...
are available OTC (usually in strictly limited formulations or requiring paperwork or identification to be submitted during purchase).
Over time, often 3–6 years, drugs that prove themselves safe and appropriate as prescription medicines may be switched from prescription to OTC. An example of this is diphenhydramine
Diphenhydramine (DPH) is an antihistamine and sedative mainly used to treat allergies, insomnia, and symptoms of the common cold. It is also less commonly used for tremor in parkinsonism, and nausea. It is taken by mouth, injected into a v ...
(Benadryl), an anti-histamine which once required a prescription but now is available OTC nearly everywhere. More recent examples are cimetidine
Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.
The development of longer-acting H2 r ...
and loratadine
Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis (hay fever) and hives. It is also available in combination with pseudoephedrine, a decongestant, known as lor ...
in the United States, and ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus ...
in Australia.
It is somewhat unusual for an OTC drug to be withdrawn from the market as a result of safety concerns, rather than market forces, though it does happen occasionally. For example, phenylpropanolamine
Phenylpropanolamine (PPA) is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to cont ...
was removed from sale in the United States over concern regarding strokes
Stroke (also known as a cerebrovascular accident (CVA) or brain attack) is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, ...
in young women. A study has been done examining consumer's perceptions about the risk of and access to nonprescription medication. The study concluded that a small percentage of consumers prefer having access to medication over potential risks of taking non-prescribed medication. Ranitidine was suspended in multiple markets due to concerns over the presence of the carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive subst ...
''N''-nitrosodimethylamine (NDMA).
In the United Kingdom, it was announced in February 2007 that Boots the Chemist would try over-the-counter sales of Viagra in stores in Manchester, England
Manchester () is a city in Greater Manchester, England. It had a population of 552,000 in 2021. It is bordered by the Cheshire Plain to the south, the Pennines to the north and east, and the neighbouring city of Salford to the west. The two ...
(previous available as prescription only). Men aged between 30 and 65 could buy four tablets after a consultation with a pharmacist
A pharmacist, also known as a chemist (Commonwealth English) or a druggist (North American and, archaically, Commonwealth English), is a healthcare professional who prepares, controls and distributes medicines and provides advice and instructi ...
.
See also
* Child-resistant packaging * Drug interactions * Generally recognized as safe and effective *Generic drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active ...
* Inverse benefit law
* Medical prescription
A prescription, often abbreviated or Rx, is a formal communication from a physician or other registered health-care professional to a pharmacist, authorizing them to dispense a specific prescription drug for a specific patient. Historica ...
* Over-the-counter counseling
* Pharmacy
Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medication, medicines. It is a miscellaneous science as it ...
* Ranitidine impurities
* Regulation of therapeutic goods
The regulation of therapeutic goods, defined as drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United States, they are regulated at the national level by a single agency. In other jurisdictions they are reg ...
References
External links
Complete list of OTC drugs
Tool Box at ConsumerMedSafety.org
at FamilyDoctor.org, maintained by the
American Academy of Family Physicians
The American Academy of Family Physicians (AAFP) was founded in 1947 to promote and maintain high-quality standards for family medicine, an offshoot of the classical general practitioner. It is headquartered in Leawood, Kansas.
AAFP is one of ...
. Contains extensive information on over-the-counter drugs and their responsible use, including specific guidance on several drug classes in question-and-answer format and information on common drug interactions.
UK Medicines and Healthcare Products Regulatory Agency list of substances on general sales list
*
National Institute on Drug Abuse
The National Institute on Drug Abuse (NIDA) is a United States federal government research institute whose mission is to "advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual a ...
:NIDA for Teens: Cough and Cold Medicine (DXM and Codeine Syrup)
{{DEFAULTSORT:Over-The-Counter Drug Drugs Pharmaceuticals policy Drug safety