Noncoordinating Anion on:
[Wikipedia]
[Google]
[Amazon]
 A revolution in this area occurred in the 1990s with the introduction of the tetrakis ,5-bis(trifluoromethyl)phenylorate ion, , commonly abbreviated as and colloquially called "BARF". This anion is far less coordinating than tetrafluoroborate, hexafluorophosphate, and perchlorate, and consequently has enabled the study of still more electrophilic cations. Related tetrahedral anions include tetrakis(pentafluorophenyl)borate , and .
A revolution in this area occurred in the 1990s with the introduction of the tetrakis ,5-bis(trifluoromethyl)phenylorate ion, , commonly abbreviated as and colloquially called "BARF". This anion is far less coordinating than tetrafluoroborate, hexafluorophosphate, and perchlorate, and consequently has enabled the study of still more electrophilic cations. Related tetrahedral anions include tetrakis(pentafluorophenyl)borate , and .
 In the bulky borates and aluminates, the
In the bulky borates and aluminates, the 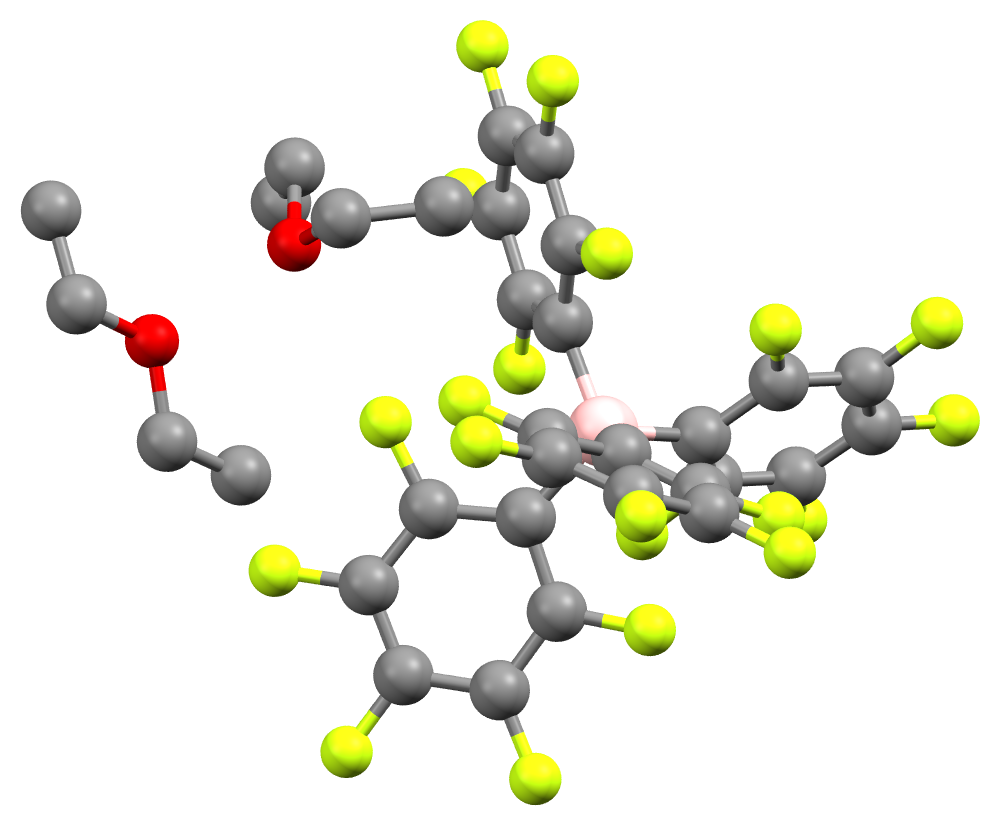 The neutral molecules that represent the parents to the non-coordinating anions are strong Lewis acids, e.g. boron trifluoride, BF3 and
The neutral molecules that represent the parents to the non-coordinating anions are strong Lewis acids, e.g. boron trifluoride, BF3 and
Anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s that interact weakly with cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
cations. They are commonly found as counterions for cationic metal complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es with an unsaturated coordination sphere. These special anions are essential components of homogeneous
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, siz ...
alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
complex. For example, they are employed as counterions for the 14 valence electron cations C5H5)2ZrRsup>+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
, hydrosilylation Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009 ...
, oligomerization
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
, and the living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
of alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s. The popularization of non-coordinating anions has contributed to increased understanding of agostic complex
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, r ...
es wherein hydrocarbons and hydrogen serve as ligands. Non-coordinating anions are important components of many superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid ...
s, which result from the combination of Brønsted acids and Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s.
Pre-"BARF" era
Before the 1990s,tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perchlo ...
(), hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . It is an octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the hexafluorosilicate dianion, , and hexafluoroantimonate . In this an ...
(), and perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. Per ...
() were considered weakly coordinating anions. These species are now known to bind to strongly electrophilic metal centers. Tetrafluoroborate and hexafluorophosphate anions are coordinating toward highly electrophilic metal ions, such as cations containing Zr(IV) centers, which can abstract fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
from these anions. Other anions, such as triflate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ' ...
s are considered to be low-coordinating with some cations.
Era of BARF
 A revolution in this area occurred in the 1990s with the introduction of the tetrakis ,5-bis(trifluoromethyl)phenylorate ion, , commonly abbreviated as and colloquially called "BARF". This anion is far less coordinating than tetrafluoroborate, hexafluorophosphate, and perchlorate, and consequently has enabled the study of still more electrophilic cations. Related tetrahedral anions include tetrakis(pentafluorophenyl)borate , and .
A revolution in this area occurred in the 1990s with the introduction of the tetrakis ,5-bis(trifluoromethyl)phenylorate ion, , commonly abbreviated as and colloquially called "BARF". This anion is far less coordinating than tetrafluoroborate, hexafluorophosphate, and perchlorate, and consequently has enabled the study of still more electrophilic cations. Related tetrahedral anions include tetrakis(pentafluorophenyl)borate , and .
 In the bulky borates and aluminates, the
In the bulky borates and aluminates, the negative charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectiv ...
is symmetrically distributed over many electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
atoms. Related anions are derived from tris(pentafluorophenyl)boron B(C6F5)3. Another advantage of these anions is that their salts are more soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
in non-polar organic solvents such as dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
, and, in some cases, even alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
s. Polar solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
s, such as acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
, THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
, and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
, tend to bind to electrophilic centers, in which cases, the use of a non-coordinating anion is pointless.
Salts of the anion were first reported by Kobayashi and co-workers. For that reason, it is sometimes referred to as ''Kobayashi's anion''. Kobayashi's method of preparation has been superseded by a safer route.
 The neutral molecules that represent the parents to the non-coordinating anions are strong Lewis acids, e.g. boron trifluoride, BF3 and
The neutral molecules that represent the parents to the non-coordinating anions are strong Lewis acids, e.g. boron trifluoride, BF3 and phosphorus pentafluoride
Phosphorus pentafluoride, P F5, is a phosphorus halide. It is a colourless, toxic gas that fumes in air.
Preparation
Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride, w ...
, PF5. A notable Lewis acid of this genre is tris(pentafluorophenyl)borane
Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound . It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the c ...
, B(C6F5)3, which abstracts alkyl ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s:
:(C5H5)2Zr(CH3)2 + B(C6F5)3 → C5H5)2Zr(CH3)sup>+ CH3)B(C6F5)3sup>−
Other types of non-coordinating anions
Another large class of non-coordinating anions are derived fromcarborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these c ...
anion . Using this anion, the first example of a three-coordinate silicon compound, the salt mesityl)3Si.html" ;"title="mesityl.html" ;"title="mesityl">mesityl)3Si">mesityl.html" ;"title="mesityl">mesityl)3SiHCB11Me5Br6] contains a non-coordinating anion derived from a carborane.
References
{{reflist Coordination chemistry Non-coordinating anions, *