Non-covalent bonding on:
[Wikipedia]
[Google]
[Amazon]
In
 Ionic interactions involve the attraction of
Ionic interactions involve the attraction of
 A
A

 Dipole–dipole interactions are electrostatic interactions between permanent
Dipole–dipole interactions are electrostatic interactions between permanent
 π–π interactions are associated with the interaction between the π-orbitals of a molecular system. The high polarizability of aromatic rings lead to dispersive interactions as major contribution to so-called stacking effects. These play a major role for interactions of nucleobases e.g. in DNA. For a simple example, a benzene ring, with its fully conjugated π cloud, will interact in two major ways (and one minor way) with a neighboring benzene ring through a π–π interaction (see figure 3). The two major ways that benzene stacks are edge-to-face, with an
π–π interactions are associated with the interaction between the π-orbitals of a molecular system. The high polarizability of aromatic rings lead to dispersive interactions as major contribution to so-called stacking effects. These play a major role for interactions of nucleobases e.g. in DNA. For a simple example, a benzene ring, with its fully conjugated π cloud, will interact in two major ways (and one minor way) with a neighboring benzene ring through a π–π interaction (see figure 3). The two major ways that benzene stacks are edge-to-face, with an
 Cation–pi interactions involve the positive charge of a
Cation–pi interactions involve the positive charge of a 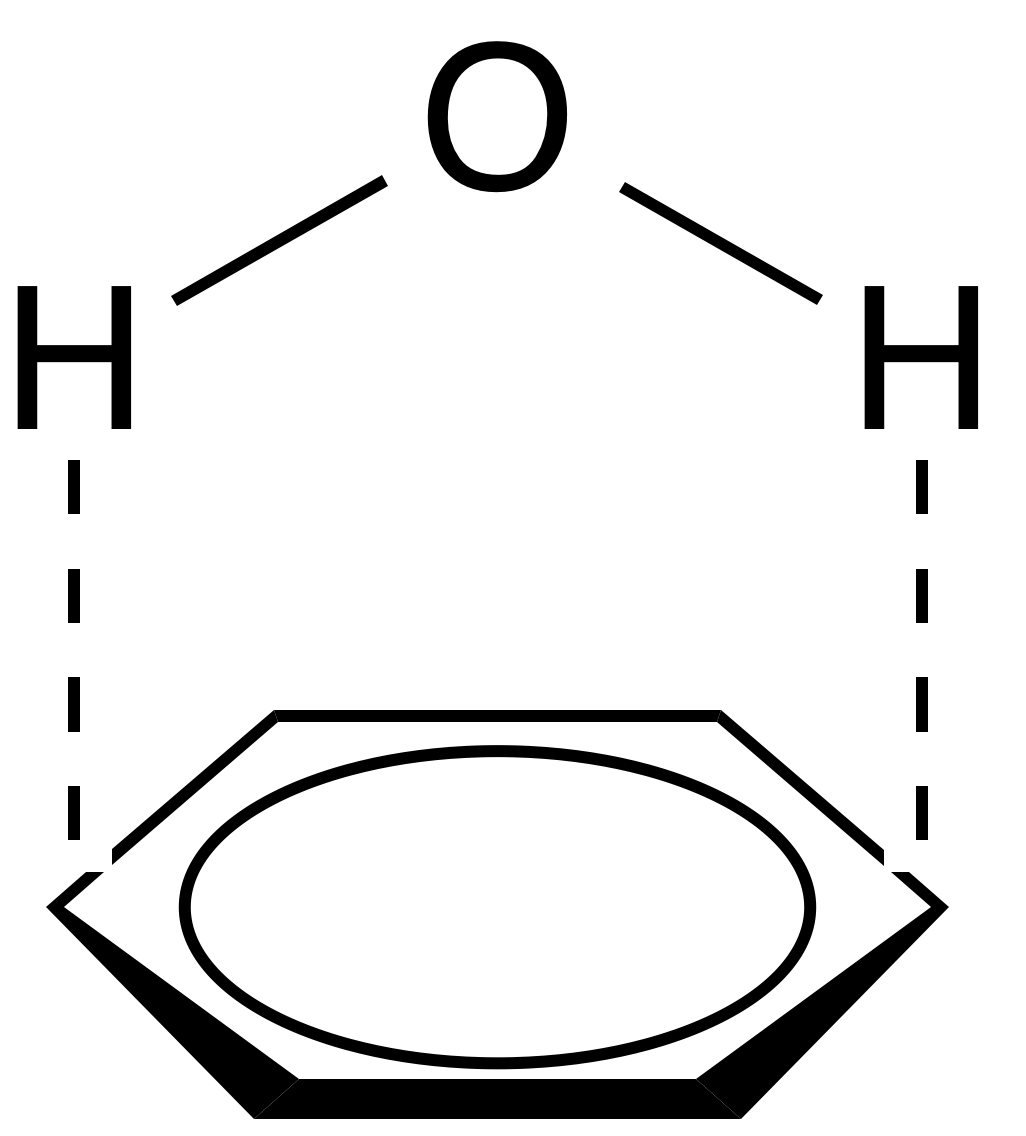
 The folding of most
The folding of most
 The predominant non-covalent interactions associated with each species in solution are listed in the above figure. As previously discussed,
The predominant non-covalent interactions associated with each species in solution are listed in the above figure. As previously discussed,
Chemical bonding Supramolecular chemistry pt:Interação não covalente
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a non-covalent interaction differs from a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
in that it does not involve the sharing of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s, but rather involves more dispersed variations of electromagnetic interaction
In physics, electromagnetism is an interaction that occurs between particles with electric charge. It is the second-strongest of the four fundamental interactions, after the strong force, and it is the dominant force in the interactions of ...
s between molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s or within a molecule. The chemical energy
Chemical energy is the energy of chemical substances that is released when they undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (2018). "How ...
released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/ mol (1000–5000 calorie
The calorie is a unit of energy. For historical reasons, two main definitions of "calorie" are in wide use. The large calorie, food calorie, or kilogram calorie was originally defined as the amount of heat needed to raise the temperature of on ...
s per 6.02 molecules). Non-covalent interactions can be classified into different categories, such as electrostatic
Electrostatics is a branch of physics that studies electric charges at rest (static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber ...
, π-effects, van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic bond, ionic or covalent bonds, these attractions do not result from a Chemical bond, chemical electronic bond; they are c ...
, and hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
s.
Non-covalent interactions are critical in maintaining the three-dimensional structure of large molecules, such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s. In addition, they are also involved in many biological processes in which large molecules bind specifically but transiently to one another (see the properties section of the DNA page). These interactions also heavily influence drug design
Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that acti ...
, crystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a big influence on hardness, density, Transparency and translucen ...
and design of materials, particularly for self-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
, and, in general, the synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
of many organic molecule
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
s.
Intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s are non-covalent interactions that occur between different molecules, rather than between different atoms of the same molecule.
Electrostatic interactions
Ionic
 Ionic interactions involve the attraction of
Ionic interactions involve the attraction of ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s or molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s with full permanent charges of opposite signs. For example, sodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is used in trace amounts in the fluoridation of drinking water, in toothpaste, in metallurgy, and as a flux. It is a colorless or white solid that is readily soluble in water. I ...
involves the attraction of the positive charge on sodium (Na+) with the negative charge on fluoride (F−). However, this particular interaction is easily broken upon addition to water, or other highly polar solvents. In water ion pairing is mostly entropy driven; a single salt bridge usually amounts to an attraction value of about ΔG =5 kJ/mol at an intermediate ion strength I, at I close to zero the value increases to about 8 kJ/mol. The ΔG values are usually additive and largely independent of the nature of the participating ions, except for transition metal ions etc.
These interactions can also be seen in molecules with a localized charge on a particular atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
. For example, the full negative charge associated with ethoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, w ...
, the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of ethanol, is most commonly accompanied by the positive charge of an alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
salt such as the sodium cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(Na+).
Hydrogen bonding
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
(H-bond), is a specific type of interaction that involves dipole–dipole attraction between a partially positive hydrogen atom and a highly electronegative, partially negative oxygen, nitrogen, sulfur, or fluorine atom (not covalently bound to said hydrogen atom). It is not a covalent bond, but instead is classified as a strong non-covalent interaction. It is responsible for why water is a liquid at room temperature and not a gas (given water's low molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
). Most commonly, the strength of hydrogen bonds lies between 0–4 kcal/mol, but can sometimes be as strong as 40 kcal/mol In solvents such as chloroform or carbon tetrachloride one observes e.g. for the interaction between amides additive values of about 5 kJ/mol. According to Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific top ...
the strength of a hydrogen bond is essentially determined by the electrostatic charges. Measurements of thousands of complexes in chloroform or carbon tetrachloride have led to additive free energy increments for all kind of donor-acceptor combinations.
Halogen bonding
Halogen bonding A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bo ...
is a type of non-covalent interaction which does not involve the formation nor breaking of actual bonds, but rather is similar to the dipole–dipole interaction
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
known as hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
. In halogen bonding, a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
atom acts as an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
, or electron-seeking species, and forms a weak electrostatic interaction with a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, or electron-rich species. The nucleophilic agent in these interactions tends to be highly electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
(such as oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, or sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
), or may be anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic, bearing a negative formal charge
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electroneg ...
. As compared to hydrogen bonding, the halogen atom takes the place of the partially positively charged hydrogen as the electrophile.
Halogen bonding should not be confused with halogen–aromatic interactions, as the two are related but differ by definition. Halogen–aromatic interactions involve an electron-rich aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
π-cloud as a nucleophile; halogen bonding is restricted to monatomic nucleophiles.
Van der Waals forces
Van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic bond, ionic or covalent bonds, these attractions do not result from a Chemical bond, chemical electronic bond; they are c ...
are a subset of electrostatic interactions involving permanent or induced dipoles (or multipoles). These include the following:
*permanent dipole–dipole interaction
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s, alternatively called the Keesom force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
*dipole-induced dipole interactions, or the Debye force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
*induced dipole-induced dipole interactions, commonly referred to as London dispersion forces
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between at ...
Hydrogen bonding and halogen bonding are typically not classified as Van der Waals forces.
Dipole–dipole
dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
s in molecules. These interactions tend to align the molecules to increase attraction (reducing potential energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors.
Common types of potential energy include the gravitational potentia ...
). Normally, dipoles are associated with electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
atoms, including oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, and fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
.
For example, acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
, the active ingredient in some nail polish removers, has a net dipole associated with the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
(see figure 2). Since oxygen is more electronegative than the carbon that is covalently bonded to it, the electrons associated with that bond will be closer to the oxygen than the carbon, creating a partial negative charge (δ−) on the oxygen, and a partial positive charge (δ+) on the carbon. They are not full charges because the electrons are still shared through a covalent bond between the oxygen and carbon. If the electrons were no longer being shared, then the oxygen-carbon bond would be an electrostatic interaction.
:
Often molecules contain dipolar groups, but have no overall dipole moment. This occurs if there is symmetry within the molecule that causes the dipoles to cancel each other out. This occurs in molecules such as tetrachloromethane
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemica ...
. Note that the dipole-dipole interaction between two individual atoms is usually zero, since atoms rarely carry a permanent dipole. See atomic dipoles.
Dipole-induced dipole
A dipole-induced dipole interaction (Debye force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
) is due to the approach of a molecule with a permanent dipole to another non-polar molecule with no permanent dipole. This approach causes the electrons of the non-polar molecule to be polarized toward or away from the dipole (or "induce" a dipole) of the approaching molecule. Specifically, the dipole can cause electrostatic attraction or repulsion of the electrons from the non-polar molecule, depending on orientation of the incoming dipole. Atoms with larger atomic radii are considered more "polarizable" and therefore experience greater attractions as a result of the Debye force.
London dispersion forces
London dispersion force
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between at ...
s are the weakest type of non-covalent interaction. In organic molecules, however, the multitude of contacts can lead to larger contributions, particularly in the presence of heteroatoms. They are also known as "induced dipole-induced dipole interactions" and present between all molecules, even those which inherently do not have permanent dipoles. Dispersive interactions increase with the polarizability of interacting groups, but are weakened by solvents of increased polarizability. They are caused by the temporary repulsion of electrons away from the electrons of a neighboring molecule, leading to a partially positive dipole on one molecule and a partially negative dipole on another molecule. Hexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relatively ...
is a good example of a molecule with no polarity or highly electronegative atoms, yet is a liquid at room temperature due mainly to London dispersion forces. In this example, when one hexane molecule approaches another, a temporary, weak partially negative dipole on the incoming hexane can polarize the electron cloud of another, causing a partially positive dipole on that hexane molecule. In absence of solvents hydrocarbons such as hexane form crystals due to dispersive forces ; the sublimation heat of crystals is a measure of the dispersive interaction. While these interactions are short-lived and very weak, they can be responsible for why certain non-polar molecules are liquids at room temperature.
π-effects
π-effects can be broken down into numerous categories, including π-π interactions, cation-π & anion-π interactions, and polar-π interactions. In general, π-effects are associated with the interactions of molecules with the π-systems of conjugated molecules such as benzene.π–π interaction
 π–π interactions are associated with the interaction between the π-orbitals of a molecular system. The high polarizability of aromatic rings lead to dispersive interactions as major contribution to so-called stacking effects. These play a major role for interactions of nucleobases e.g. in DNA. For a simple example, a benzene ring, with its fully conjugated π cloud, will interact in two major ways (and one minor way) with a neighboring benzene ring through a π–π interaction (see figure 3). The two major ways that benzene stacks are edge-to-face, with an
π–π interactions are associated with the interaction between the π-orbitals of a molecular system. The high polarizability of aromatic rings lead to dispersive interactions as major contribution to so-called stacking effects. These play a major role for interactions of nucleobases e.g. in DNA. For a simple example, a benzene ring, with its fully conjugated π cloud, will interact in two major ways (and one minor way) with a neighboring benzene ring through a π–π interaction (see figure 3). The two major ways that benzene stacks are edge-to-face, with an enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
of ~2 kcal/mol, and displaced (or slip stacked), with an enthalpy of ~2.3 kcal/mol. The sandwich configuration is not nearly as stable of an interaction as the previously two mentioned due to high electrostatic repulsion of the electrons in the π orbitals.
Cation–π and anion–π interaction
 Cation–pi interactions involve the positive charge of a
Cation–pi interactions involve the positive charge of a cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
interacting with the electrons in a π-system of a molecule. This interaction is surprisingly strong (as strong or stronger than H-bonding in some contexts), and has many potential applications in chemical sensors. For example, the sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
ion can easily sit atop the π cloud of a benzene molecule, with ''C6'' symmetry (See figure 4).
Anion–π interactions are very similar to cation–π interactions, but reversed. In this case, an anion sits atop an electron-poor π-system, usually established by the placement of electron-withdrawing substituents on the conjugated molecule

Polar–π
Polar–π interactions involve molecules with permanent dipoles (such as water) interacting with the quadrupole moment of a π-system (such as that in benzene (see figure 5). While not as strong as a cation-π interaction, these interactions can be quite strong (~1-2 kcal/mol), and are commonly involved in protein folding and crystallinity of solids containing both hydrogen bonding and π-systems. In fact, any molecule with a hydrogen bond donor (hydrogen bound to a highly electronegative atom) will have favorable electrostatic interactions with the electron-rich π-system of a conjugated molecule.Hydrophobic effect
Thehydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
is the desire for non-polar molecules to aggregate in aqueous solutions in order to separate from water. This phenomenon leads to minimum exposed surface area of non-polar molecules to the polar water molecules (typically spherical droplets), and is commonly used in biochemistry to study protein folding and other various biological phenomenon. The effect is also commonly seen when mixing various oils (including cooking oil) and water. Over time, oil sitting on top of water will begin to aggregate into large flattened spheres from smaller droplets, eventually leading to a film of all oil sitting atop a pool of water. However the hydrophobic effect is not considered a non-covalent interaction as it is a function of entropy and not a specific interaction between two molecules, usually characterized by entropy.enthalpy compensation. An essentially enthalpic hydrophobic effect materializes if a limited number of water molecules are restricted within a cavity; displacement of such water molecules by a ligand frees the water molecules which then in the bulk water enjoy a maximum of hydrogen bonds close to four.
Examples
Drug design
Most pharmaceutical drugs are small molecules which elicit a physiological response by "binding" toenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s or receptors
Receptor may refer to:
*Sensory receptor, in physiology, any structure which, on receiving environmental stimuli, produces an informative nerve impulse
*Receptor (biochemistry), in biochemistry, a protein molecule that receives and responds to a n ...
, causing an increase or decrease in the enzyme's ability to function. The binding of a small molecule to a protein is governed by a combination of steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
, or spatial considerations, in addition to various non-covalent interactions, although some drugs do covalently modify an active site (see irreversible inhibitors). Using the "lock and key model" of enzyme binding, a drug (key) must be of roughly the proper dimensions to fit the enzyme's binding site (lock). Using the appropriately sized molecular scaffold, drugs must also interact with the enzyme non-covalently in order to maximize binding affinity binding constant
The binding constant, or affinity constant/association constant, is a special case of the equilibrium constant ''K'', and is the inverse of the dissociation constant. It is associated with the binding and unbinding reaction of receptor (R) and li ...
and reduce the ability of the drug to dissociate from the binding site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may inclu ...
. This is achieved by forming various non-covalent interactions between the small molecule and amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
in the binding site, including: hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
, electrostatic interactions
Electrostatics is a branch of physics that studies electric charges at Rest (physics), rest (static electricity).
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles af ...
, pi stacking
In chemistry, pi stacking (also called π–π stacking) refers to the presumptive attractive, noncovalent pi interactions ( orbital overlap) between the pi bonds of aromatic rings. However this is a misleading description of the phenomena sinc ...
, van der Waals interactions, and dipole–dipole interaction
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s.
Non-covalent metallo drugs have been developed. For example, dinuclear triple-helical compounds in which three ligand strands wrap around two metals, resulting in a roughly cylindrical tetracation have been prepared. These compounds bind to the less-common nucleic acid structures, such as duplex DNA, Y-shaped fork structures and 4-way junctions.
Protein folding and structure
 The folding of most
The folding of most proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
from a primary (linear) sequence of amino acids to a three-dimensional structure is governed by many factors, including non-covalent interactions. The first ~5 milliseconds of folding are primarily dependent on hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
, whereby the protein folds so as to orient nonpolar amino acids in the interior of the globular protein, while more polar amino acid residues are exposed to aqueous solvent. This phase is known as the hydrophobic collapse
Hydrophobic collapse is a proposed process for the production of the Protein structure, 3-D conformation adopted by Peptide, polypeptides and other molecules in polar solvents. The theory states that the nascent polypeptide forms initial Protein se ...
, when nonpolar non-covalent interactions exclude water from the interior of the developing 3D protein structure.
After this initial "burst phase," more polar non-covalent interactions take over. Between 5 and 1000 milliseconds after protein folding initiation, three-dimensional structures of proteins, known as secondary
Secondary may refer to: Science and nature
* Secondary emission, of particles
** Secondary electrons, electrons generated as ionization products
* The secondary winding, or the electrical or electronic circuit connected to the secondary winding i ...
and tertiary structures
Biomolecular structure is the intricate folded, three-dimensional shape that is formed by a molecule of protein, DNA, or RNA, and that is important to its function. The structure of these molecules may be considered at any of several length s ...
, are stabilized by formation of hydrogen bonds, in addition to disulfide bridges
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
(covalent linkages). Through a series of small conformational changes, spatial orientations are modified so as to arrive at the most energetically minimized orientation achievable. The folding of proteins is often facilitated by enzymes known as molecular chaperones
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to ass ...
. Sterics
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
, bond strain, and angle strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are ...
also play major roles in the folding of a protein from its primary sequence to its tertiary structure.
Single tertiary protein structures can also assemble to form protein complexes composed of multiple independently folded subunits. As a whole, this is called a protein's quaternary structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also refe ...
. The quaternary structure is generated by the formation of relatively strong non-covalent interactions, such as hydrogen bonds, between different subunits to generate a functional polymeric enzyme. Some proteins also utilize non-covalent interactions to bind cofactors
Cofactor may also refer to:
* Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed
* A domain parameter in elliptic curve cryptography, defined as the ratio between the order ...
in the active site during catalysis, however a cofactor can also be covalently attached to an enzyme. Cofactors can be either organic or inorganic molecules which assist in the catalytic mechanism of the active enzyme. The strength with which a cofactor is bound to an enzyme may vary greatly; non-covalently bound cofactors are typically anchored by hydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
or electrostatic interactions
Electrostatics is a branch of physics that studies electric charges at Rest (physics), rest (static electricity).
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles af ...
.
Boiling points
Non-covalent interactions have a significant effect on theboiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
of a liquid. Boiling point is defined as the temperature at which the vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
of a liquid is equal to the pressure surrounding the liquid. More simply, it is the temperature at which a liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
becomes a gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
. As one might expect, the stronger the non-covalent interactions present for a substance, the higher its boiling point. For example, consider three compounds of similar chemical composition: sodium n-butoxide (C4H9ONa), diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
(C4H10O), and n-butanol
1-Butanol, also known as butan-1-ol or ''n''-butanol, is a primary alcohol with the chemical formula C4H9OH and a linear structure. Isomers of 1-butanol are isobutanol, butan-2-ol and ''tert''-butanol. The unmodified term butanol usually refers ...
(C4H9OH).
 The predominant non-covalent interactions associated with each species in solution are listed in the above figure. As previously discussed,
The predominant non-covalent interactions associated with each species in solution are listed in the above figure. As previously discussed, ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
ic interactions require considerably more energy to break than hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, which in turn are require more energy than dipole–dipole interaction
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s. The trends observed in their boiling points (figure 8) shows exactly the correlation expected, where sodium n-butoxide requires significantly more heat energy (higher temperature) to boil than n-butanol, which boils at a much higher temperature than diethyl ether. The heat energy required for a compound to change from liquid to gas is associated with the energy required to break the intermolecular forces
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
each molecule experiences in its liquid state.
References
{{Chemical bondsChemical bonding Supramolecular chemistry pt:Interação não covalente