Magic Number (physics) on:
[Wikipedia]
[Google]
[Amazon]
 In
In 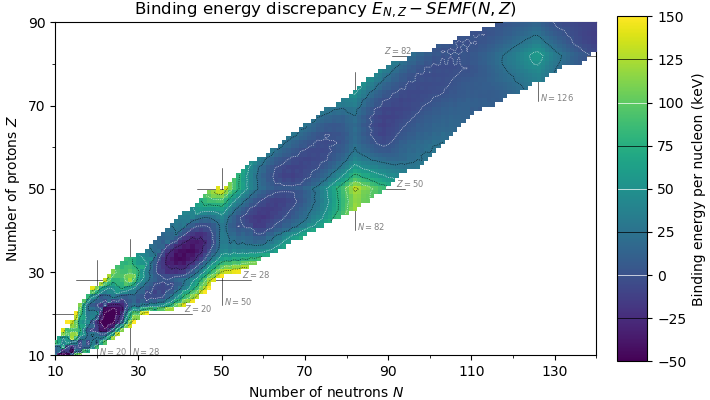 Before this was realized, higher magic numbers, such as 184, 258, 350, and 462 , were predicted based on simple
calculations that assumed spherical shapes: these are generated by the formula (see
Before this was realized, higher magic numbers, such as 184, 258, 350, and 462 , were predicted based on simple
calculations that assumed spherical shapes: these are generated by the formula (see
 Upon working on the
Upon working on the
nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies the ...
, a magic number is a number of nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons were ...
s (either proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s or neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s, separately) such that they are arranged into complete shells within the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron i ...
. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126 .
For protons, this corresponds to the elements helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
and the hypothetical unbihexium
Unbihexium, also known as element 126 or eka-plutonium, is the hypothetical chemical element with atomic number 126 and placeholder symbol Ubh. ''Unbihexium'' and ''Ubh'' are the temporary systematic element name, IUPAC name and symbol, respecti ...
, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
per nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons were ...
than one would expect based upon predictions such as the semi-empirical mass formula
In nuclear physics, the semi-empirical mass formula (SEMF) (sometimes also called the Weizsäcker formula, Bethe–Weizsäcker formula, or Bethe–Weizsäcker mass formula to distinguish it from the Bethe–Weizsäcker process) is used to approx ...
and are hence more stable against nuclear decay.
The unusual stability of isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s having magic numbers means that transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s could theoretically be created with extremely large nuclei and yet not be subject to the extremely rapid radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
normally associated with high atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
s. Large isotopes with magic numbers of nucleons are said to exist in an island of stability. Unlike the magic numbers 2–126, which are realized in spherical nuclei, theoretical calculations predict that nuclei in the island of stability are deformed.
 Before this was realized, higher magic numbers, such as 184, 258, 350, and 462 , were predicted based on simple
calculations that assumed spherical shapes: these are generated by the formula (see
Before this was realized, higher magic numbers, such as 184, 258, 350, and 462 , were predicted based on simple
calculations that assumed spherical shapes: these are generated by the formula (see Binomial coefficient
In mathematics, the binomial coefficients are the positive integers that occur as coefficients in the binomial theorem. Commonly, a binomial coefficient is indexed by a pair of integers and is written \tbinom. It is the coefficient of the t ...
). It is now believed that the sequence of spherical magic numbers cannot be extended in this way. Further predicted magic numbers are 114, 122, 124, and 164 for protons as well as 184, 196, 236, and 318 for neutrons.
However, more modern calculations predict 228 and 308 for neutrons, along with 184 and 196.
History and etymology
 Upon working on the
Upon working on the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
, the German physicist Maria Goeppert Mayer
Maria Goeppert Mayer (; June 28, 1906 – February 20, 1972) was a German-born American theoretical physicist, and Nobel laureate in Physics for proposing the nuclear shell model of the atomic nucleus. She was the second woman to win a Nobel Pr ...
became interested in the properties of nuclear fission products, such as decay energies and half-lives. In 1948, she published a body of experimental evidence for the occurrence of closed nuclear shells for nuclei with 50 or 82 protons or 50, 82, and 126 neutrons.
It had already been known that nuclei with 20 protons or neutrons were stable: that was evidenced by calculations by Hungarian-American physicist Eugene Wigner
Eugene Paul "E. P." Wigner ( hu, Wigner Jenő Pál, ; November 17, 1902 – January 1, 1995) was a Hungarian-American theoretical physicist who also contributed to mathematical physics. He received the Nobel Prize in Physics in 1963 "for his co ...
, one of her colleagues in the Manhattan Project. Two years later, in 1950, a new publication followed in which she attributed the shell closures at the magic numbers to spin-orbit coupling.
According to Steven Moszkowski (a student of Maria Goeppert Mayer), the term "magic number" was coined by Wigner: "Wigner too believed in the liquid drop model
In nuclear physics, the semi-empirical mass formula (SEMF) (sometimes also called the Weizsäcker formula, Bethe–Weizsäcker formula, or Bethe–Weizsäcker mass formula to distinguish it from the Bethe–Weizsäcker process) is used to approxi ...
, but he recognized, from the work of Maria Mayer, the very strong evidence for the closed shells. It seemed a little like magic to him, and that is how the words 'Magic Numbers' were coined."
These magic numbers were the bedrock of the nuclear shell model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model is a model of the atomic nucleus which uses the Pauli exclusion principle to describe the structure of the nucleus in terms of energy levels. The first shell m ...
, which Mayer developed in the following years together with Hans Jensen and culminated in their shared 1963 Nobel Prize in Physics.
Doubly magic
Nuclei which have neutron number and proton ( atomic) numbers each equal to one of the magic numbers are called "doubly magic", and are especially stable against decay. The known doubly magic isotopes arehelium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consis ...
, helium-10, oxygen-16
Oxygen-16 (16O) is a stable isotope of oxygen, having 8 neutrons and 8 protons in its nucleus. It has a mass of . Oxygen-16 is the most abundant isotope of oxygen and accounts for 99.762% of oxygen's natural abundance. The relative and absol ...
, calcium-40
Calcium (20Ca) has 26 known isotopes, ranging from 35Ca to 60Ca. There are five stable isotopes (40Ca, 42Ca, 43Ca, 44Ca and 46Ca), plus one isotope ( 48Ca) with such a long half-life that for all practical purposes it can be considered stable. T ...
, calcium-48
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
-48, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
-56, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
-78, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
-100, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
-132, and lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
-208. However, only the first, third, fourth, and last of these doubly magic nuclides are completely stable, although calcium-48 is extremely long-lived and therefore naturally occurring, disintegrating only by a very inefficient double beta minus decay process. Double beta decay in general is so rare that several nuclides exist which are predicted to decay by this mechanism but in which no such decay has yet been observed. Even in nuclides whose double beta decay has been confirmed through observations, half lives usually exceed the age of the universe
In physical cosmology, the age of the universe is the time elapsed since the Big Bang. Astronomers have derived two different measurements of the age of the universe:
a measurement based on direct observations of an early state of the universe, ...
by orders of magnitude and emitted beta- or gamma radiation is for virtually all practical purposes irrelevant.
Doubly-magic effects may allow existence of stable isotopes which otherwise would not have been expected. An example is calcium-40
Calcium (20Ca) has 26 known isotopes, ranging from 35Ca to 60Ca. There are five stable isotopes (40Ca, 42Ca, 43Ca, 44Ca and 46Ca), plus one isotope ( 48Ca) with such a long half-life that for all practical purposes it can be considered stable. T ...
, with 20 neutrons and 20 protons, which is the heaviest stable isotope made of the same number of protons and neutrons. Both calcium-48
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
-48 are doubly magic because calcium-48 has 20 protons and 28 neutrons while nickel-48 has 28 protons and 20 neutrons. Calcium-48 is very neutron-rich for such a relatively light element, but like calcium-40, it is stabilized by being doubly magic.
Magic number shell effects are seen in ordinary abundances of elements: helium-4 is among the most abundant (and stable) nuclei in the universe and lead-208 is the heaviest stable nuclide. Alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
(the emission of a He-4 nucleus - also known as an alpha particle - by a heavy element undergoing radioactive decay) is common in part due to the extraordinary stability of Helium-4 which makes this type of decay energetically favored in most heavy nuclei over neutron emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and ...
, proton emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case t ...
or any type of cluster decay other than He-4 emission. The stability of He-4 also leads to the absence of stable isobars of mass number 5 and 8 - indeed all nuclides of those mass numbers decay within fractions of a second to produce alpha particles.
Magic effects can keep unstable nuclides from decaying as rapidly as would otherwise be expected. For example, the nuclides tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
-100 and tin-132 are examples of doubly magic isotopes of tin
Tin (50Sn) is the element with the greatest number of stable isotopes (ten; three of them are potentially radioactive but have not been observed to decay), which is probably related to the fact that 50 is a " magic number" of protons. Twenty-nin ...
that are unstable, and represent endpoints beyond which stability drops off rapidly. Nickel-48, discovered in 1999, is the most proton-rich doubly magic nuclide known. At the other extreme, nickel-78 is also doubly magic, with 28 protons and 50 neutrons, a ratio observed only in much heavier elements, apart from tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus o ...
with one proton and two neutrons (78Ni: 28/50 = 0.56; 238U: 92/146 = 0.63).
In December 2006, hassium
Hassium is a chemical element with the symbol Hs and the atomic number 108. Hassium is highly radioactive; its most stable known isotopes have half-lives of approximately ten seconds. One of its isotopes, 270Hs, has magic numbers of both protons ...
-270, with 108 protons and 162 neutrons, was discovered by an international team of scientists led by the Technical University of Munich
The Technical University of Munich (TUM or TU Munich; german: Technische Universität München) is a public research university in Munich, Germany. It specializes in engineering, technology, medicine, and applied and natural sciences.
Establis ...
, having a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 9 seconds. Hassium-270 evidently forms part of an island of stability, and may even be doubly magic due to the deformed (American football
American football (referred to simply as football in the United States and Canada), also known as gridiron, is a team sport played by two teams of eleven players on a rectangular field with goalposts at each end. The offense, the team with ...
- or rugby ball
A rugby ball is an elongated ellipsoidal ball used in both codes of rugby football. Its measurements and weight are specified by World Rugby and the Rugby League International Federation, the governing bodies for both codes, rugby union and rugby ...
-like) shape of this nucleus.
Although ''Z'' = 92 and ''N'' = 164 are not magic numbers, the undiscovered neutron-rich nucleus uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
-256 may be doubly magic and spherical due to the difference in size between low- and high-angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
orbitals, which alters the shape of the nuclear potential
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nucl ...
.
Derivation
Magic numbers are typically obtained by empirical studies; if the form of thenuclear potential
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nucl ...
is known, then the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
can be solved for the motion of nucleons and energy levels determined. Nuclear shells are said to occur when the separation between energy levels is significantly greater than the local mean separation.
In the shell model
The SHELL model is a conceptual model of human factors that clarifies the scope of aviation human factors and assists in understanding the human factor relationships between aviation system resources/environment (the flying subsystem) and the huma ...
for the nucleus, magic numbers are the numbers of nucleons at which a shell is filled. For instance, the magic number 8 occurs when the 1s1/2, 1p3/2, 1p1/2 energy levels are filled, as there is a large energy gap between the 1p1/2 and the next highest 1d5/2 energy levels.
The atomic analog to nuclear magic numbers are those numbers of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s leading to discontinuities in the ionization energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
. These occur for the noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
es helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, neon, argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
, krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often ...
, xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
, radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through ...
and oganesson
Oganesson is a synthetic chemical element with the symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint team of Russian and American scient ...
. Hence, the "atomic magic numbers" are 2, 10, 18, 36, 54, 86 and 118. As with the nuclear magic numbers, these are expected to be changed in the superheavy region due to spin–orbit coupling effects affecting subshell energy levels. Hence copernicium
Copernicium is a synthetic chemical element with the symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of ap ...
(112) and flerovium
Flerovium is a Transactinide element, superheavy chemical element with Chemical symbol, symbol Fl and atomic number 114. It is an extremely radioactive synthetic element. It is named after the Flerov Laboratory of Nuclear Reactions of the Joint ...
(114) are expected to be more inert than oganesson (118), and the next noble gas after these is expected to occur at element 172 rather than 168 (which would continue the pattern).
In 2010, an alternative explanation of magic numbers was given in terms of symmetry considerations. Based on the fractional extension of the standard rotation group, the ground state properties (including the magic numbers) for metallic clusters and nuclei were simultaneously determined analytically. A specific potential term is not necessary in this model.
See also
* Magic number (chemistry) * Superatom * SuperdeformationReferences
External links
* * * see chapter 10 especially. * * {{DEFAULTSORT:Physics - Magic Number Isotopes Periodic table Radioactivity Integer sequences