Multiple Reaction Monitoring on:
[Wikipedia]
[Google]
[Amazon]
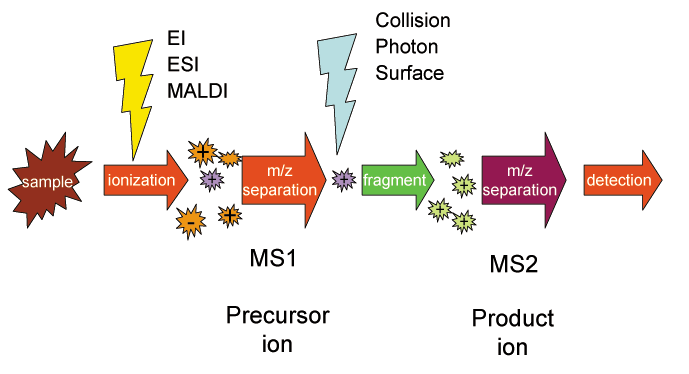 Selected reaction monitoring (SRM), also called Multiple reaction monitoring, (MRM), is a method used in tandem mass spectrometry in which an
Selected reaction monitoring (SRM), also called Multiple reaction monitoring, (MRM), is a method used in tandem mass spectrometry in which an
SRMatlas
quantify proteins in complex proteome digests by mass spectrometry {{Quantitative proteomics Mass spectrometry Proteomics
 Selected reaction monitoring (SRM), also called Multiple reaction monitoring, (MRM), is a method used in tandem mass spectrometry in which an
Selected reaction monitoring (SRM), also called Multiple reaction monitoring, (MRM), is a method used in tandem mass spectrometry in which an ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
of a particular mass is selected in the first stage of a tandem mass spectrometer and an ion product of a fragmentation reaction of the precursor ions is selected in the second mass spectrometer stage for detection.
Variants
A general case of SRM can be represented by : where the precursor ion ABCD+ is selected by the first stage of mass spectrometry (MS1), dissociates into molecule AB and product ion CD+, and the latter is selected by the second stage of mass spectrometry (MS2) and detected. The precursor and product ion pair is called a SRM "transition." Consecutive reaction monitoring (CRM) is the serial application of three or more stages of mass spectrometry to SRM, represented in a simple case by : where ABCD+ is selected by MS1, dissociates into molecule AB and ion CD+. The ion is selected in the second mass spectrometry stage MS2 then undergoes further fragmentation to form ion D+ which is selected in the third mass spectrometry stage MS3 and detected. Multiple reaction monitoring (MRM) is the application of selected reaction monitoring to multiple product ions from one or more precursor ions, for example : : where ABCD+ is selected by MS1 and dissociates by two pathways, forming either AB+ or CD+. The ions are selected sequentially by MS2 and detected. Parallel reaction monitoring (PRM) is the application of SRM with parallel detection of all transitions in a single analysis using a high resolution mass spectrometer.Proteomics
SRM can be used for targeted quantitative proteomics bymass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
. Following ionization in, for example, an electrospray
The name electrospray is used for an apparatus that employs electricity to disperse a liquid or for the fine aerosol resulting from this process. High voltage is applied to a liquid supplied through an emitter (usually a glass or metallic capilla ...
source, a peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
precursor is first isolated to obtain a substantial ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
population of mostly the intended species. This population is then fragmented to yield product ions whose signal abundances are indicative of the abundance of the peptide in the sample. This experiment can be performed on triple quadrupole mass spectrometers
A triple quadrupole mass spectrometer (TQMS), is a tandem mass spectrometry, tandem mass spectrometer consisting of two quadrupole mass analyzers in series, with a (non-mass-resolving) Radio-frequency quadrupole, radio frequency (RF)–only quadru ...
, where mass-resolving Q1 isolates the precursor, q2 acts as a collision cell, and mass-resolving Q3 is cycled through the product ions which are detected upon exiting the last quadrupole by an electron multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an ele ...
. A precursor/product pair is often referred to as a ''transition''. Much work goes into ensuring that transitions are selected that have maximum specificity.
Using isotopic labeling with heavy-labeled (e.g., D, 13C, or 15N) peptides to a complex matrix as concentration standards, SRM can be used to construct a calibration curve
In analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. ...
that can provide the absolute quantification (i.e., copy number per cell) of the native, light peptide, and by extension, its parent protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
.
SRM has been used to identify the proteins encoded by wild-type and mutant genes (mutant proteins
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mit ...
) and quantify their absolute copy numbers in tumors and biological fluids, thus answering the basic questions about the absolute copy number of proteins in a single cell, which will be essential in digital modelling of mammalian cells and human body, and the relative levels of genetically abnormal proteins in tumors, and proving useful for diagnostic applications. SRM has also been used as a method of triggering full product ion scans of peptides to either a) confirm the specificity of the SRM transition, or b) detect specific post-translational modifications
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes ...
which are below the limit of detection of standard MS analyses. In 2017, SRM has been developed to be a highly sensitive and reproducible mass spectrometry-based protein targeted detection platform (entitled "SAFE-SRM"), and it has been demonstrated that the SRM-based new pipeline has major advantages in clinical proteomics applications over traditional SRM pipelines, and it has demonstrated a dramatically improved diagnostic performance over that from antibody-based protein biomarker diagnostic methods, such as ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence ...
.
See also
* Quantitative proteomics *Protein mass spectrometry
Protein mass spectrometry refers to the application of mass spectrometry to the study of proteins. Mass spectrometry is an important method for the accurate mass determination and characterization of proteins, and a variety of methods and instrume ...
References
External links
SRMatlas
quantify proteins in complex proteome digests by mass spectrometry {{Quantitative proteomics Mass spectrometry Proteomics