Molecular sensor on:
[Wikipedia]
[Google]
[Amazon]
 A molecular sensor or chemosensor is a molecular structure (organic or inorganic complexes) that is used for sensing of an
A molecular sensor or chemosensor is a molecular structure (organic or inorganic complexes) that is used for sensing of an  Chemosensors describes molecule of synthetic origin that signal the presence of matter or energy. A chemosensor can be considered as type of an analytical device. Chemosensors are used in everyday life and have been applied to various areas such as in chemistry, biochemistry, immunology, physiology, etc. and within medicine in general, such as in critical care analysis of blood samples. Chemosensors can be designed to detect/signal a single analyte or a mixture of such species in solution. This can be achieved through either a single measurement or through the use of continuous monitoring. The signalling moiety acts as a
Chemosensors describes molecule of synthetic origin that signal the presence of matter or energy. A chemosensor can be considered as type of an analytical device. Chemosensors are used in everyday life and have been applied to various areas such as in chemistry, biochemistry, immunology, physiology, etc. and within medicine in general, such as in critical care analysis of blood samples. Chemosensors can be designed to detect/signal a single analyte or a mixture of such species in solution. This can be achieved through either a single measurement or through the use of continuous monitoring. The signalling moiety acts as a  Chemosensors were first used to describe the combination of a molecular recognition with some form of reporter so the presence of a guest can be observed (also referred to as the analyte, c.f. above). Chemosensors are designed to contain a signalling moiety and a molecular recognition moiety (also called the binding site or a receptor). Combining both of these components can be achieved in a number of ways, such as integrated, twisted or spaced. Chemosensors are consider as major component of the area of
Chemosensors were first used to describe the combination of a molecular recognition with some form of reporter so the presence of a guest can be observed (also referred to as the analyte, c.f. above). Chemosensors are designed to contain a signalling moiety and a molecular recognition moiety (also called the binding site or a receptor). Combining both of these components can be achieved in a number of ways, such as integrated, twisted or spaced. Chemosensors are consider as major component of the area of  Optical signalling methods (such as
Optical signalling methods (such as
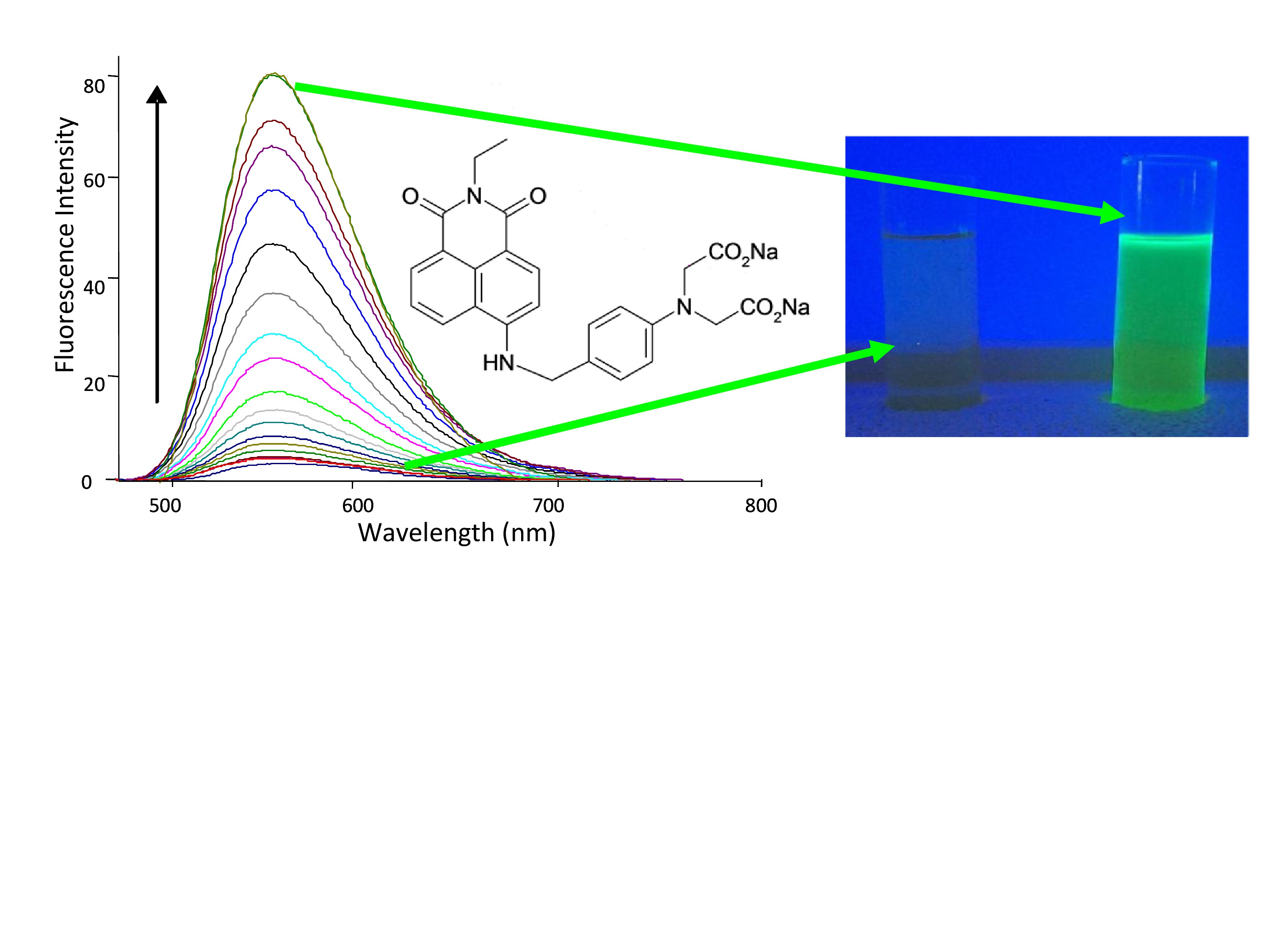 The fluorophores-receptor can also be integrated within the chemosensor. This leads to changes in the emission wavelength, which often results in change in colour. When the sensing event results in the formation of a signal that is visible to the naked eye, such sensors are normally referred to as
The fluorophores-receptor can also be integrated within the chemosensor. This leads to changes in the emission wavelength, which often results in change in colour. When the sensing event results in the formation of a signal that is visible to the naked eye, such sensors are normally referred to as
 Chemosensors have been incorporated through surface functionalization onto particles and beads such as metal based
Chemosensors have been incorporated through surface functionalization onto particles and beads such as metal based  The compound saxitoxin is a neurotoxin found in shellfish and a chemical weapon. An experimental sensor for this compound is again based on PET. Interaction of saxitoxin with the sensor's crown ether moiety kills its PET process towards the fluorophore and fluorescence is switched from off to on. The unusual boron moiety makes sure the fluorescence takes place in the visible light part of the electromagnetic spectrum.
The compound saxitoxin is a neurotoxin found in shellfish and a chemical weapon. An experimental sensor for this compound is again based on PET. Interaction of saxitoxin with the sensor's crown ether moiety kills its PET process towards the fluorophore and fluorescence is switched from off to on. The unusual boron moiety makes sure the fluorescence takes place in the visible light part of the electromagnetic spectrum.
 A molecular sensor or chemosensor is a molecular structure (organic or inorganic complexes) that is used for sensing of an
A molecular sensor or chemosensor is a molecular structure (organic or inorganic complexes) that is used for sensing of an analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
to produce a detectable change or a signal
In signal processing, a signal is a function that conveys information about a phenomenon. Any quantity that can vary over space or time can be used as a signal to share messages between observers. The '' IEEE Transactions on Signal Processing' ...
. The action of a chemosensor, relies on an interaction occurring at the molecular level, usually involves the continuous monitoring of the activity of a chemical species in a given matrix such as solution, air, blood, tissue, waste effluents, drinking water, etc. The application of chemosensors is referred to as chemosensing, which is a form of molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, halogen ...
. All chemosensors are designed to contain a signalling moiety and a recognition moiety, that is connected either directly to each other or through a some kind of connector or a spacer. The signalling is often optically based electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible ...
, giving rise to changes in either (or both) the ultraviolet and visible absorption or the emission
Emission may refer to:
Chemical products
* Emission of air pollutants, notably:
**Flue gas, gas exiting to the atmosphere via a flue
** Exhaust gas, flue gas generated by fuel combustion
** Emission of greenhouse gases, which absorb and emit radi ...
properties of the sensors. Chemosensors may also be electrochemically based. Small molecule sensors
Small molecule sensors are an effective way to detect the presence of metal ions in solution. Although many types exist, most small molecule sensors comprise a subunit that selectively binds to a metal that in turn induces a change in a fluorescen ...
are related to chemosensors. These are traditionally, however, considered as being structurally simple molecules and reflect the need to form chelating
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
molecules for complexing ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
in analytical chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separati ...
. Chemosensors are synthetic analogues of biosensors, the difference being that biosensors incorporate biological receptors such as antibodies, aptamers or large biopolymers.
 Chemosensors describes molecule of synthetic origin that signal the presence of matter or energy. A chemosensor can be considered as type of an analytical device. Chemosensors are used in everyday life and have been applied to various areas such as in chemistry, biochemistry, immunology, physiology, etc. and within medicine in general, such as in critical care analysis of blood samples. Chemosensors can be designed to detect/signal a single analyte or a mixture of such species in solution. This can be achieved through either a single measurement or through the use of continuous monitoring. The signalling moiety acts as a
Chemosensors describes molecule of synthetic origin that signal the presence of matter or energy. A chemosensor can be considered as type of an analytical device. Chemosensors are used in everyday life and have been applied to various areas such as in chemistry, biochemistry, immunology, physiology, etc. and within medicine in general, such as in critical care analysis of blood samples. Chemosensors can be designed to detect/signal a single analyte or a mixture of such species in solution. This can be achieved through either a single measurement or through the use of continuous monitoring. The signalling moiety acts as a signal transducer
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellula ...
, converting the information (recognition event between the chemosensor and the analyte) into an optical response in a clear and reproducible manner.
Most commonly, the change (the signal) is observed by measuring the various physical properties of the chemosensor, such as the photo-physical properties seen in the absorption or emission
Emission may refer to:
Chemical products
* Emission of air pollutants, notably:
**Flue gas, gas exiting to the atmosphere via a flue
** Exhaust gas, flue gas generated by fuel combustion
** Emission of greenhouse gases, which absorb and emit radi ...
, where different wavelengths of the electromagnetic spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from ...
are used. Consequently, most chemosensors are described as being either colorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color ...
( ground state) or luminescent
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crysta ...
(excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers t ...
, fluorescent
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, th ...
or phosphorescent). Colorimetric chemosensors give rise to changes in their absorption properties (recorded using ultraviolet–visible spectroscopy
UV spectroscopy or UV–visible spectrophotometry (UV–Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relative ...
), such as in absorption intensity and wavelength or in chirality (using circularly polarized light
In electrodynamics, circular polarization of an electromagnetic wave is a polarization state in which, at each point, the electromagnetic field of the wave has a constant magnitude and is rotating at a constant rate in a plane perpendicular to t ...
, and CD spectroscopy).
In contrast, then in the case of luminescent chemosensors, the detection of an analyte, using fluorescence spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the elect ...
, gives rise to spectral changes in the fluorescence excitation or in the emission spectra, which are recorded using a fluorimeter. Such changes can also occur in other excited state properties such as in the excited state life-time(s), quantum yield of fluorescence, and polarisation, etc. of the chemosensor. Fluorescence detection can be achieved at a low concentration (below ~ 10-6 M) with most fluorescence spectrometers. This offers the advantage of using the sensors directly within fibre optic systems. Examples of the use of chemosensors are to monitor blood content, drug concentrations, etc., as well as in environmental samples. Ions and molecules occur in abundance in biological and environmental systems where they are involved/effete biological and chemical processes. The development of molecular chemosensors as probes for such analytes is an annual multibillion-dollar business involving both small SMEs as well as large pharmaceutical and chemical companies.
 Chemosensors were first used to describe the combination of a molecular recognition with some form of reporter so the presence of a guest can be observed (also referred to as the analyte, c.f. above). Chemosensors are designed to contain a signalling moiety and a molecular recognition moiety (also called the binding site or a receptor). Combining both of these components can be achieved in a number of ways, such as integrated, twisted or spaced. Chemosensors are consider as major component of the area of
Chemosensors were first used to describe the combination of a molecular recognition with some form of reporter so the presence of a guest can be observed (also referred to as the analyte, c.f. above). Chemosensors are designed to contain a signalling moiety and a molecular recognition moiety (also called the binding site or a receptor). Combining both of these components can be achieved in a number of ways, such as integrated, twisted or spaced. Chemosensors are consider as major component of the area of molecular diagnostics
Molecular diagnostics is a collection of techniques used to analyze biological markers in the genome and proteome, and how their cells express their genes as proteins, applying molecular biology to medical testing. In medicine the technique i ...
, within the discipline of supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular force ...
, which relies on molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, halogen ...
. In terms of supramolecular chemistry, chemosensing is an example of host–guest chemistry In supramolecular chemistry, host–guest chemistry describes inclusion compound, complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bond ...
, where the presence of a guest (the analyte) at the host site (the sensor) gives rise to recognition event (e.g. sensing) that can be monitored in real time. This requires the binding of the analyte to the receptor, using all kinds of binding interactions such as hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
, dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system ...
- and electrostatic interactions, solvophobic effect, metal chelation, etc. The recognition/binding moiety is responsible for selectivity and efficient binding of the guest/analyte, which depend on ligand topology, characteristics of the target (ionic radius, size of molecule, chirality, charge, coordination number and hardness, etc.) and the nature of the solvent (pH, ionic strength, polarity). Chemosensors are normally developed to be able to interact with the target species in reversible manner, which is a prerequisite for continuous monitoring.
 Optical signalling methods (such as
Optical signalling methods (such as fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, ...
) are sensitive and selective, and provide a platform for real-time response, and local observation. As chemosensors are designed to be both targeting (i.e. can recognize and bind a specific species) and sensitive to various concentration ranges, they can be used to observed real-live events on the cellular level. As each molecule can give rise to a signal/readout, that can be selectively measured, chemosensors are often said to be non-invasive and consequently have attracted significant attentions for their applications within biological matter, such as within living cells. Many examples of chemosensors have been developed for observing cellular function and properties, including monitoring ion flux concentrations and transports within cells such as Ca(II), Zn(II), Cu(II) and other physiologically important cations and anions, as well as biomolecules.
The design of ligands for the selective recognition of suitable guests such as metal cations
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
and anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
has been an important goal of supramolecular chemistry. The term supramolecular analytical chemistry has recently been coined to describe the application of molecular sensors to analytical chemistry. Small molecule sensors
Small molecule sensors are an effective way to detect the presence of metal ions in solution. Although many types exist, most small molecule sensors comprise a subunit that selectively binds to a metal that in turn induces a change in a fluorescen ...
are related to chemosensors. However, these are traditionally considered as being structurally simple molecules and reflect the need to form chelating molecules for complexing ions in analytical chemistry.
History
While chemosensors were first defined in the 1980s, the first example of such a fluorescent chemosensor can be documented to be that of Friedrich Goppelsroder, who in 1867, developed a method for the determination/sensing of aluminium ion, using fluorescent ligand/chelate. This and subsequent work by others, gave birth to what is considered as modern analytical chemistry. In the 1980s the development of chemosensing was achieved by Anthony W. Czarnik, A. Prasanna de Silva andRoger Tsien
Roger Yonchien Tsien (pronounced , "'' CHEN''"'';'' February 1, 1952 – August 24, 2016) was an American biochemist. He was a professor of chemistry and biochemistry at the University of California, San Diego and was awarded the Nobel Prize in ...
, who developed various types of luminescent probes for ions and molecules in solutions and within biological cells, for real-time applications. Tsien went on to study and developing this area of research further by developing and studding fluorescent proteins for applications in biology, such as green fluorescent protein
The green fluorescent protein (GFP) is a protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. The label ''GFP'' traditionally refers to the protein first isolated from the jellyfish '' Aeq ...
s (GFP) for which he was awarded the Nobel Prize in Chemistry in 2008. The work of Lynn Sousa in the late 1970s, on the detection of alkali metal ions, possibly resulting in one of the first examples of the use of supramolecular chemistry in fluorescent sensing design, as well as that of J.-M. Lehn, H. Bouas-Laurent and co-workers at Université Bordeaux I, France. The development of PET sensing of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
ions was developed by L. Fabbrizzi, among others.
In chemosensing, the use of fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with ...
connected to the receptor via a covalent spacer is now commonly referred to as fluorophores-spacer-receptor principle. In such systems, the sensing event is normally described as being due to changes in the photophysical properties of the chemosensor systems due to chelation induced enhanced fluorescence (CHEF), and photoinduced electron transfer
Photoinduced electron transfer (PET) is an excited state electron transfer process by which an excited electron is transferred from donor to acceptor."Organic and Inorganic Photochemistry" V. Ramamurthy and Kirk S. Schanze 1998 Marcel Dekker Due ...
(PET), mechanisms. In principle the two mechanisms are based on the same idea; the communication pathway is in the form of a through-space electron transfer from the electron rich receptors to the electron deficient fluorophores (through space). This results in fluorescence quenching (active electron transfer), and the emission from the chemosensor is 'switched off,' for both mechanisms in the absence of the analytes. However, upon forming a host–guest complex between the analyte and receptor, the communication pathway is broken and the fluorescence emission from the fluorophores is enhanced, or 'switched on'. In other words, the fluorescence intensity and quantum yield are enhanced upon analyte recognition.
 The fluorophores-receptor can also be integrated within the chemosensor. This leads to changes in the emission wavelength, which often results in change in colour. When the sensing event results in the formation of a signal that is visible to the naked eye, such sensors are normally referred to as
The fluorophores-receptor can also be integrated within the chemosensor. This leads to changes in the emission wavelength, which often results in change in colour. When the sensing event results in the formation of a signal that is visible to the naked eye, such sensors are normally referred to as colorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color ...
. Many examples of colorimetric chemosensors for ions such as fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts ...
have been developed. A pH indicator
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hen ...
can be consider as a colorimetric chemosensors for protons. Such sensors have been developed for other cations, as well as anions and larger organic and biological molecules, such as proteins and carbohydrates.
Design principles
Chemosensors are nano-sized molecules and for applicationin vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and ...
need to be non-toxic. A chemosensor must be able to give a measurable signal in direct response to the analyte recognition. Hence, the signal response is directly related to the magnitude of the sensing event (and, in turn concentration of the analyte). While the signalling moiety acts as a signal transducer, converting the recognition event into an optical response. The recognition moiety is responsible for binding to the analyte in a selective and reversible manner. If the binding sites are 'irreversible chemical reactions,' the indicators are described as fluorescent chemodosimeters, or fluorescent probes
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with sev ...
.
An active communication pathway has to be open between the two moieties for the sensor to operate. In colorimetric chemosensors, this usually relies on the receptor and transducer to be structurally integrated. In luminescent/fluorescent chemosensing these two parts can be 'spaced' out or connected with a covalent spacer. The communication pathway is through electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons.
Electrochemical processes ar ...
or energy transfer
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat ...
for such fluorescent chemosensors. The effectiveness of the host–guest recognition between the receptor and the analyte depends on several factors, including the design of the receptor moiety, which is objective is to match as much the nature of the structural nature of the target analyte, as well as the nature of the environment that the sensing event occurs within (e.g. the type of media, i.e. blood, saliva, urine, etc. in biological samples). An extension to this approach is the development of molecular beacons, which are oligonucleotide hybridization probes based on fluorescence signalling where the recognition or the sensing event is communicated through enhancement or reduction in luminescence through the use of Förster resonance energy transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). ...
(FRET) mechanism.
Fluorescent chemosensing
All chemosensors are designed to contain a signalling moiety and a recognition moiety. These are integrated directly or connected with a short covalent spacer depending on the mechanism involved in the signalling event. The chemosensor can be based onself-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
of the sensor and the analyte. An example of such a design are the (indicator) displacement assays IDA. IDA sensor for anions such as citrate or phosphate ions have been developed whereby these ions can displace a fluorescent indicator in an indicator-host complex. The so-called UT taste chip (University of Texas) is a prototype electronic tongue and combines supramolecular chemistry with charge-coupled devices based on silicon wafers and immobilized receptor molecules.
Most examples of chemosensors for ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
, such as those of alkali metal ions (Li+, Na+, K+, etc.) and alkali earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...
ions (Mg2+, Ca2+, etc.) are designed so that the excited state of the fluorophore component of the chemosensor is quenched by an electron transfer when the sensor is not complexed to these ions. No emission is thus observed, and the sensor is sometimes referred to as being 'switched off'. By complexing the sensor with a cation, the conditions for electron transfer are altered so that the quenching process is blocked, and fluorescence emission is 'switched on'. The probability of PET is governed by the overall free energy of the system (the Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work (physics), work that may be performed by a closed system, thermodynamically closed system a ...
ΔG). The driving force for PET is represented by ΔGET, the overall changes in the free energy for the electron transfer can be estimated using the Rehm-Weller equation. Electron transfer is distance dependent and decreases with increasing spacer length. Quenching by electron transfer between uncharged species leads to the formation of a radical ion pair. This is sometimes referred to as being the primary electron transfer. The possible electron transfer, which takes place after the PET, is referred to as the 'secondary electron transfer'. Chelation Enhancement Quenching (CHEQ) is the opposite effect seen for CHEF. In CHEQ, a reduction is observed in fluorescent emission of the chemosensor in comparison to that seen the originally for the 'free' sensor upon host–guest formation. As electron transfer is directional, such systems have also been described by the PET principle, being described as an enhancement in PET from the receptor to the fluorophore with enhanced degree of quenching. Such an effect has been demonstrated for the sensing of anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
such as carboxylates and fluorides.
A large number of examples of chemosensors have been developed by scientists in physical, life and environmental sciences. The advantages of fluorescence emission being 'switched on' from 'off' upon the recognition event enabling the chemosensors to be compared to 'beacons in the night'. As the process is reversible, the emission enhancement is concentration dependent, only becoming 'saturated' at high concentrations (fully bound receptor). Hence, a correlation can be made between luminescence (intensity, quantum yield and in some cases lifetime) and the analyte concentration. Through careful design, and evaluation of the nature of the communication pathway, similar sensors based on the use of 'on-off' switching, or 'on-off-on,' or 'off-on-off' switching have been designed. The incorporation of chemosensors onto surfaces, such as quantum dots, nanoparticles
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
, or into polymers
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic an ...
is also a fast-growing area of research. Other examples of chemosensors that work on the principle of switching fluorescent emission either on or off include, Förster resonance energy transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). ...
(FRET), internal charge transfer (ICT), twisted internal charge transfer (TICT), metal-based emission (such as in lanthanide luminescence), and excimer and exciplex emission and aggregation-induced emission
Aggregation-induced emission (AIE) is a phenomenon that is observed with certain organic luminophores (fluorescent dyes).
The photoemission efficiencies of most organic compounds is higher in solution than in the solid state. Photoemission fro ...
(AIE). Chemosensors were one of the first examples of molecules that could result in switching between 'on' or 'off' states through the use of external stimuli and as such can be classed as synthetic molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for ...
, to which the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
was awarded to in 2016 to Jean-Pierre Sauvage
Jean-Pierre Sauvage (; born 21 October 1944) is a French coordination chemist working at Strasbourg University. He graduated from the National School of Chemistry of Strasbourg (now known as ECPM Strasbourg), in 1967. He has specialized in s ...
, Fraser Stoddart and Bernard L. Feringa
Bernard Lucas Feringa (, born 18 May 1951) is a Dutch synthetic organic chemist, specializing in molecular nanotechnology and homogeneous catalysis. He is the Jacobus van 't Hoff Distinguished Professor of Molecular Sciences, at the Stratingh ...
.
The application of these same design principles used in chemosensing also paved the way for the development of molecular logic gates
A molecular logic gate is a molecule that performs a logical operation based on one or more physical or chemical inputs and a single output. The field has advanced from simple logic systems based on a single chemical or physical input to molecules ...
mimics (MLGMs), being first proposed using PET based fluorescent chemosensors by de Silva and co-workers in 1993. Molecules have been made to operate in accordance with Boolean algebra
In mathematics and mathematical logic, Boolean algebra is a branch of algebra. It differs from elementary algebra in two ways. First, the values of the variables are the truth values ''true'' and ''false'', usually denoted 1 and 0, whereas ...
that performs a logical operation based on one or more physical or chemical inputs. The field has advanced from the development of simple logic systems based on a single chemical input to molecules capable of carrying out complex and sequential operations.
Applications of Chemosensors
 Chemosensors have been incorporated through surface functionalization onto particles and beads such as metal based
Chemosensors have been incorporated through surface functionalization onto particles and beads such as metal based nanoparticles
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
, quantum dot
Quantum dots (QDs) are semiconductor particles a few nanometres in size, having optical and electronic properties that differ from those of larger particles as a result of quantum mechanics. They are a central topic in nanotechnology. When the q ...
s, carbon-based particles and into soft materials such as polymers to facilitate their various applications.
Other receptors are sensitive not to a specific molecule but to a molecular compound class, these chemosensors are used in array- (or microarray) based sensors. Array-based sensors utilise analyte binding by the differential receptors. One example is the grouped analysis of several tannic acids that accumulate in ageing Scotch whisky in oak barrels. The grouped results demonstrated a correlation with the age but the individual components did not. A similar receptor can be used to analyze tartrates in wine.
The application of chemosensors in cellular imaging is particularly promising as most biological process are now monitored by using imaging technologies such as confocal fluorescence and superresolution microscopy, among others.
 The compound saxitoxin is a neurotoxin found in shellfish and a chemical weapon. An experimental sensor for this compound is again based on PET. Interaction of saxitoxin with the sensor's crown ether moiety kills its PET process towards the fluorophore and fluorescence is switched from off to on. The unusual boron moiety makes sure the fluorescence takes place in the visible light part of the electromagnetic spectrum.
The compound saxitoxin is a neurotoxin found in shellfish and a chemical weapon. An experimental sensor for this compound is again based on PET. Interaction of saxitoxin with the sensor's crown ether moiety kills its PET process towards the fluorophore and fluorescence is switched from off to on. The unusual boron moiety makes sure the fluorescence takes place in the visible light part of the electromagnetic spectrum.
See also
* Boronic acids in supramolecular chemistry: Saccharide recognition *Host–guest chemistry In supramolecular chemistry, host–guest chemistry describes inclusion compound, complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bond ...
* Molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for ...
* Molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, halogen ...
References
{{Reflist Supramolecular chemistry Molecular machines