MoOPH on:
[Wikipedia]
[Google]
[Amazon]
MoOPH, also known as oxodiperoxymolybdenum(pyridine)-(hexamethylphosphoric triamide), is a
 Due to MoOPH's
Due to MoOPH's  In addition,
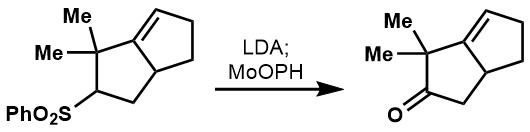
In addition,  In the case of sulfones, alpha-hydroxylation leads directly to the ketone or aldehyde.
In the case of sulfones, alpha-hydroxylation leads directly to the ketone or aldehyde.
 Common byproducts of the alpha-hydroxylation tend to include overoxidation to the corresponding dicarbonyl or intermolecular aldol reaction of the starting material. Procedures to prevent side reactions include the inverse addition of the enolate to MoOPH or careful control of the temperature (-78 to -20 °C). Notable miscellaneous reactions include MoOPH’s ability to oxidize alkylboranes directly to the alcohol with net stereo-retention.
Common byproducts of the alpha-hydroxylation tend to include overoxidation to the corresponding dicarbonyl or intermolecular aldol reaction of the starting material. Procedures to prevent side reactions include the inverse addition of the enolate to MoOPH or careful control of the temperature (-78 to -20 °C). Notable miscellaneous reactions include MoOPH’s ability to oxidize alkylboranes directly to the alcohol with net stereo-retention.
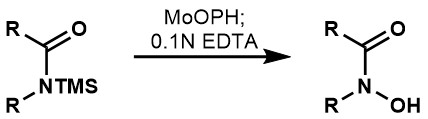
 MoOPH has also been shown to oxidize N-trimethylsilyl amides directly to the hydroxamic acid.
MoOPH has also been shown to oxidize N-trimethylsilyl amides directly to the hydroxamic acid.
reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
used in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. It contains a molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
(VI) center with multiple oxygen ligands, coordinated with pyridine and HMPA ligands. It is an electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
source of oxygen that reacts with enolates and related structures, and thus can be used for alpha-hydroxylation
In chemistry, hydroxylation can refer to:
*(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound.
*(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a ...
of carbonyl-containing compounds. Other reagents used for alpha-hydroxylation via enol or enolate structures include Davis oxaziridine
Davis reagent (3-phenyl-2-(phenylsulfonyl)-1,2-oxaziridine or 2-(benzenesulfonyl)-3-phenyloxaziridine) is a reagent used for oxidation in the Davis oxidation reaction, as well as oxidation of thiols to sulfones. It is named for Franklin A. Davis.
...
, oxygen, and various peroxyacid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the perox ...
s (see Rubottom oxidation). This reagent was first utilized by Edwin Vedejs
Edwin Vedejs () ( lv, Edvīns Vedējs; January 31, 1941 – December 2, 2017) was a Latvian-American professor of chemistry. In 1967, he joined the organic chemistry faculty at University of Wisconsin. He rose through the ranks during his 32 years ...
as an efficient alpha-hydroxylating agent in 1974 and an effective preparative procedure was later published in 1978.
Synthesis
MoOPH is synthesized from molybdenum trioxide by oxidation with hydrogen peroxide and addition of the HMPA and pyridine ligands: :Reactivity
 Due to MoOPH's
Due to MoOPH's steric bulk
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
, preferential attack at the O–O bond occurs from the less hindered enolate face in the absence of stereoelectronic
An electronic effect influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between t ...
factors.
nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
s with acidic alpha protons can be converted directly to cyanohydrins; however, in the case of branched nitriles, this reaction directly affords the ketone.
 In the case of sulfones, alpha-hydroxylation leads directly to the ketone or aldehyde.
In the case of sulfones, alpha-hydroxylation leads directly to the ketone or aldehyde.
 Common byproducts of the alpha-hydroxylation tend to include overoxidation to the corresponding dicarbonyl or intermolecular aldol reaction of the starting material. Procedures to prevent side reactions include the inverse addition of the enolate to MoOPH or careful control of the temperature (-78 to -20 °C). Notable miscellaneous reactions include MoOPH’s ability to oxidize alkylboranes directly to the alcohol with net stereo-retention.
Common byproducts of the alpha-hydroxylation tend to include overoxidation to the corresponding dicarbonyl or intermolecular aldol reaction of the starting material. Procedures to prevent side reactions include the inverse addition of the enolate to MoOPH or careful control of the temperature (-78 to -20 °C). Notable miscellaneous reactions include MoOPH’s ability to oxidize alkylboranes directly to the alcohol with net stereo-retention.
 MoOPH has also been shown to oxidize N-trimethylsilyl amides directly to the hydroxamic acid.
MoOPH has also been shown to oxidize N-trimethylsilyl amides directly to the hydroxamic acid.

References
{{reflist Reagents for organic chemistry Molybdenum(VI) compounds Pyridine complexes Phosphoramides Peroxides