|
Rubottom Oxidation
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product.Kürti, pp. 388–389.Myers, A.G. Chemistry 215: Oxidation . chem.harvard.eduLi, pp. 478–479.Chen, B. C.; Zhou, P.; Davis, F. A.; Ciganek, E. (2003) “α-Hydroxylation of Enolates and Silyl Enol Ethers." in ''Organic Reactions''; Ed. Overman, L.E. Wiley, Chapter 1, pp. 1–355, . The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylrhenium Trioxide
Methylrhenium trioxide, also known as methyltrioxorhenium(VII), is an organometallic compound with the formula CH3ReO3. It is a volatile, colourless solid that has been used as a catalyst in some laboratory experiments. In this compound, rhenium has a tetrahedral coordination geometry with one methyl and three oxo ligands. The oxidation state of rhenium is +7. Synthesis Methylrhenium trioxide is commercially available. It can be prepared by many routes, a typical method is the reaction of rhenium heptoxide and tetramethyltin: :Re2O7 + (CH3)4Sn → CH3ReO3 + (CH3)3SnOReO3 Analogous alkyl and aryl derivatives are known. Compounds of the type RReO3 are Lewis acids, forming both 1:1 and 1:2 adducts with halides and amines. Uses Methylrhenium trioxide serves as a heterogeneous catalyst for a variety of transformations. Supported on alumina/silica, it catalyzes olefin metathesis at 25 °C. In solution, MTO catalyses for the oxidations with hydrogen peroxide. Terminal al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Bis(trimethylsilyl)amide
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula . It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species. Preparation LiHMDS is commercially available, but it can also be prepared by the deprotonation of bis(trimethylsilyl)amine with ''n''-butyllithium. This reaction can be performed ''in situ''. : Once formed, the compound can be purified by sublimation or distillation. Reactions and applications As a base LiHMDS is often used in organic chemistry as a strong non-nucleophilic base. Its conjugate acid has a p''K''a of ~26, making it is less basic than other lithium bases, such as LDA (p''K''a of conjugate acid ~36), but it is more sterically hindered and hence less nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
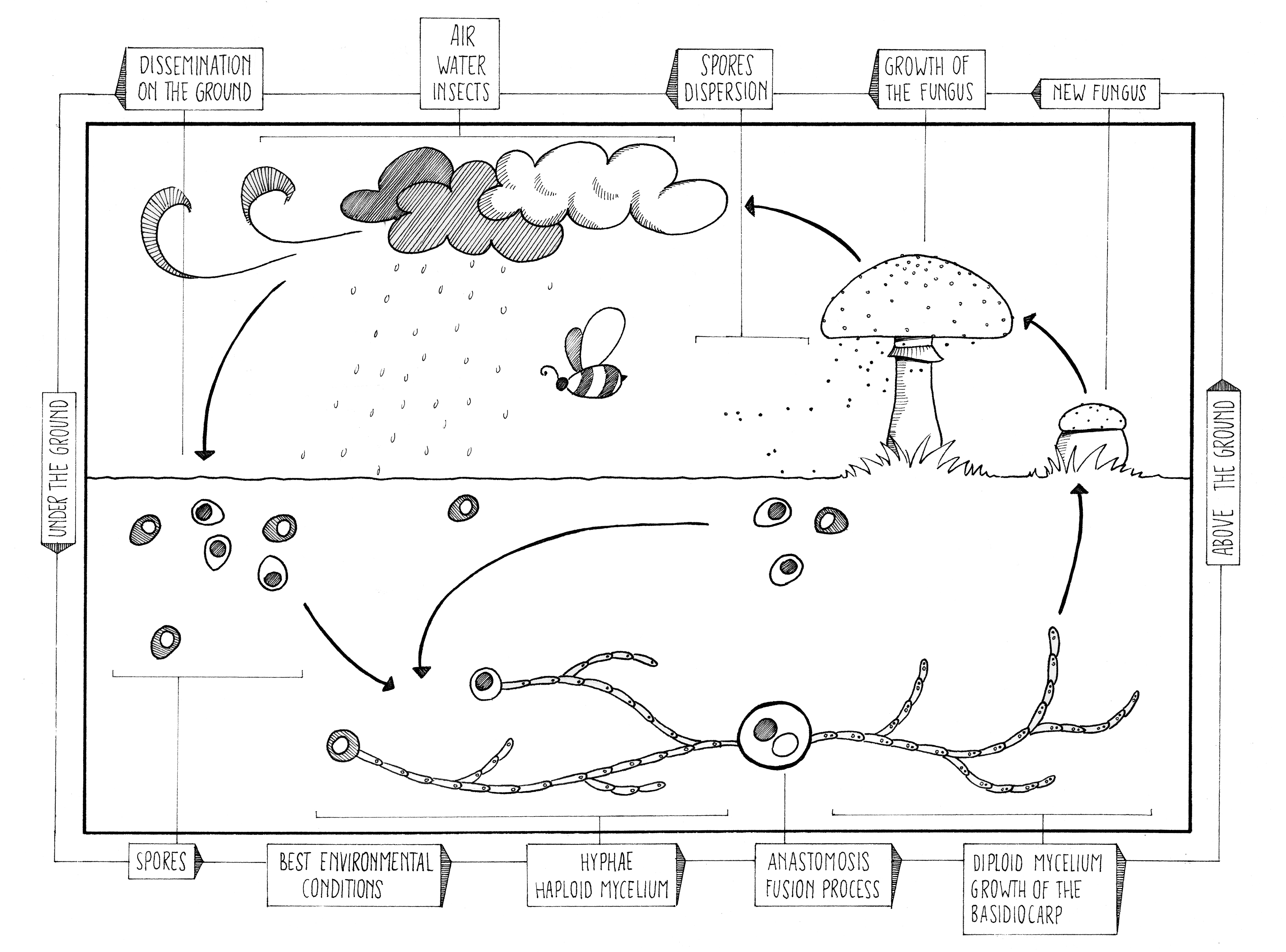
Basidiomycota
Basidiomycota () is one of two large divisions that, together with the Ascomycota, constitute the subkingdom Dikarya (often referred to as the "higher fungi") within the kingdom Fungi. Members are known as basidiomycetes. More specifically, Basidiomycota includes these groups: mushrooms, puffballs, stinkhorns, bracket fungi, other polypores, jelly fungi, boletes, chanterelles, earth stars, smuts, bunts, rusts, mirror yeasts, and ''Cryptococcus'', the human pathogenic yeast. Basidiomycota are filamentous fungi composed of hyphae (except for basidiomycota-yeast) and reproduce sexually via the formation of specialized club-shaped end cells called basidia that normally bear external meiospores (usually four). These specialized spores are called basidiospores. However, some Basidiomycota are obligate asexual reproducers. Basidiomycota that reproduce asexually (discussed below) can typically be recognized as members of this division by gross similarity to others, by the form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eric Jacobsen (chemist)
Eric N. Jacobsen (born February 22, 1960, in New York City, New York) is the Sheldon Emery Professor of Chemistry and former Chair of the Department of Chemistry and Chemical Biology at Harvard University. He is a prominent figure in the field of organic chemistry and is best known for the development of the Jacobsen epoxidation and other work in selective catalysis. Early life and education Jacobsen was born on February 22, 1960, in New York City. Jacobsen attended New York University for his undergraduate studies, graduating with his B.S. in 1982. He attended the University of California, Berkeley for graduate school, earning his Ph.D. in 1986 under the tutelage of Robert G. Bergman. He subsequently joined the laboratory of Barry Sharpless, then at MIT, as an NIH Postdoctoral Fellow. He began his independent career as an assistant professor at the University of Illinois at Urbana-Champaign in 1988. In 1993 he moved to Harvard as a full Professor. Notable contributions Jaco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-crown-6
18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is in cyclohexane and in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen. Synthesis This compound is prepared by a modified Williamson ether synthesis in the presence of a templating cation: It can be also prepared by the oligomerization of ethylene oxide: :(CH2OCH2CH2Cl)2 + (CH2OCH2CH2OH)2 + 2 KOH → (CH2CH2O)6 + 2 KCl + 2 H2O It can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold commercially as a slurry (~35%) in mineral oil or sometimes paraffin wax to facilitate dispensing. Preparation Potassium hydride is produced by direct combination of the metal and hydrogen: : This reaction was discovered by Humphry Davy soon after his 1807 discovery of potassium, when he noted that the metal would vaporize in a current of hydrogen when heated just below its boiling point.Humphry Davy (1808), ''The Bakerian Lecture on some new phenomena of chemical changes produced by electricity, particularly the decomposition of fixed alkalies, and the exhibition of the new substances which constitute their bases; and on the general nature of alkaline bodies.'' Philosophical Transactions of the Royal Society, volume 88, pages 1–44. In ''Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
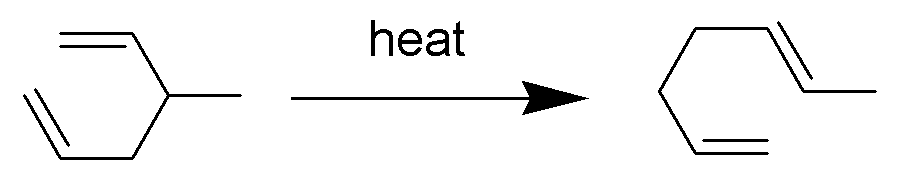
Cope Rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene. The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family. Mechanism The Cope rearrangement is the prototypical example of a concerted sigmatropic rearrangement. It is classified as a ,3sigmatropic rearrangement with the Woodward–Hoffmann symbol π2s+σ2s+π2s.html" ;"title="sub>π2s+σ2s+π2s">sub>π2s+σ2s+π2sand is therefore thermally allowed. It is sometimes useful to think of it as going through a transition state energetically and structurally equivalent to a diradical, although the diradical is not usually a true intermediate (potential energy minimum). The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |